How are ketones produced by the liver?
Here’s a step-by-step explanation of how ketones are produced:
- Glucose depletion: When glucose levels are low, either due to fasting or a low-carbohydrate diet, the body’s primary source of energy is limited. This triggers a hormonal response, including a decrease in insulin levels and an increase in glucagon and epinephrine.
- Lipolysis: In response to the hormonal changes, stored fats (triglycerides) in adipose tissue are broken down into free fatty acids and glycerol in a process called lipolysis.
- Fatty acid oxidation: Free fatty acids are transported to the liver, where they undergo a series of chemical reactions known as fatty acid oxidation or beta-oxidation. This process converts fatty acids into molecules called acetyl-CoA.
- Acetyl-CoA conversion: Under normal conditions, acetyl-CoA would enter the citric acid cycle (also known as the Krebs cycle) within the mitochondria of liver cells to produce energy. However, in ketogenesis, acetyl-CoA is converted into ketone bodies instead.
- Ketone body synthesis: The liver cells contain specific enzymes that convert excess acetyl-CoA into three types of ketone bodies: acetoacetate, beta-hydroxybutyrate, and acetone. Acetoacetate is the primary ketone body formed, while beta-hydroxybutyrate is derived from acetoacetate.
- Ketone release: Once synthesized, ketone bodies are released into the bloodstream, where they can be transported to other tissues, such as the brain, muscle, and heart, to be used as an alternative fuel source.
It’s important to note that ketone production is a normal physiological process that occurs to some extent even in individuals who consume a standard diet. However, ketone levels increase significantly during fasting or carbohydrate-restricted diets, leading to a state called nutritional ketosis. In certain pathological conditions like diabetic ketoacidosis, ketone production can become excessive, leading to a potentially dangerous buildup of ketones in the blood.
Here’s a simplified explanation of the fat oxidation process:
- Mobilization of fatty acids: When the body needs energy, signals such as low glucose levels or increased energy demands trigger the release of hormones like glucagon and epinephrine. These hormones activate hormone-sensitive lipase, an enzyme that breaks down stored triglycerides in adipose tissue into glycerol and free fatty acids.
- Activation and transport: Free fatty acids released from adipose tissue are then transported through the bloodstream, bound to a carrier protein called albumin. The fatty acids reach target tissues, including liver cells and muscle cells, where they are taken up.
- Entry into mitochondria: Once inside the cells, fatty acids undergo a series of reactions to prepare them for oxidation. They are activated by attaching a molecule called coenzyme A (CoA) to their structure, forming fatty acyl-CoA. This process occurs in the outer mitochondrial membrane.
- Beta-oxidation: Fatty acyl-CoA molecules move into the mitochondria’s inner compartment, known as the mitochondrial matrix. The process of beta-oxidation occurs here, where the fatty acyl-CoA molecules are broken down into two-carbon units called acetyl-CoA. This process involves a repetitive sequence of four steps: oxidation, hydration, oxidation, and thiolysis.
- Acetyl-CoA utilization: The acetyl-CoA generated from beta-oxidation enters the citric acid cycle (also known as the Krebs cycle) within the mitochondria. In the citric acid cycle, acetyl-CoA is further metabolized, leading to the production of ATP (the energy currency of the cell) and other intermediate molecules.
Overall, fat oxidation provides a continuous supply of energy when glucose levels are low, such as during fasting, prolonged exercise, or a low-carbohydrate diet. It is an essential process for maintaining energy balance and meeting the body’s energy demands.
Insulin: Insulin is a hormone produced and released by the beta cells of the pancreas. Its primary function is to lower blood sugar levels by promoting the uptake and utilization of glucose by cells throughout the body. Here’s how insulin works:
- Glucose uptake: When blood glucose levels rise, such as after a meal, the pancreas releases insulin into the bloodstream. Insulin binds to receptors on the surface of cells, particularly in muscle, adipose tissue, and the liver. This binding triggers the activation of glucose transporters (mainly GLUT4) on the cell membrane, allowing glucose to enter the cells.

- Glycogen synthesis: Once inside the cells, glucose is used for immediate energy needs, but excess glucose is converted into glycogen through a process called glycogenesis. Insulin stimulates glycogen synthesis in the liver and muscle cells, leading to the storage of glucose for later use.
- Inhibition of gluconeogenesis: Insulin also suppresses the production of new glucose through gluconeogenesis, which primarily occurs in the liver. By inhibiting key enzymes involved in gluconeogenesis, insulin helps prevent excessive glucose production and release into the bloodstream.
- Lipid synthesis and storage: Insulin promotes the synthesis of fatty acids and their conversion into triglycerides in adipose tissue. It also inhibits the breakdown of stored fats (lipolysis). These actions promote the storage of excess energy in the form of fat.
Glucagon: Glucagon is a hormone produced by the alpha cells of the pancreas, and its primary role is to increase blood sugar levels when they are too low. It acts in opposition to insulin. Here’s how glucagon functions:
- Glycogen breakdown: When blood glucose levels drop, such as during fasting or between meals, the pancreas releases glucagon. Glucagon binds to receptors on liver cells, initiating a signaling cascade that leads to the breakdown of glycogen (glycogenolysis) into glucose. The released glucose enters the bloodstream, raising blood sugar levels.
- Gluconeogenesis: Glucagon also promotes the synthesis of glucose through gluconeogenesis in the liver. It stimulates the conversion of amino acids, lactate, and glycerol into glucose, ensuring a continuous supply of glucose for the body’s energy needs.
- Ketone production: Glucagon indirectly stimulates the production of ketone bodies in the liver, as it promotes the breakdown of fatty acids (lipolysis) and the subsequent conversion of acetyl-CoA into ketone bodies through ketogenesis.
Overall, insulin and glucagon work in a delicate balance to regulate blood sugar levels. Insulin promotes glucose uptake and storage, while glucagon stimulates glucose production and mobilization from glycogen stores to maintain glucose homeostasis in the body.

Chronic stress can have significant effects on the regulation of insulin and glucagon, potentially leading to imbalances in blood sugar control. Here’s how chronic stress can impact these hormones:
- Insulin resistance: Prolonged exposure to stress, especially chronic psychological stress, has been associated with the development of insulin resistance. Insulin resistance occurs when cells become less responsive to the effects of insulin, resulting in decreased glucose uptake from the bloodstream. Stress-related factors like elevated cortisol levels and inflammation contribute to insulin resistance. As a result, the pancreas may produce more insulin to compensate for the reduced effectiveness, leading to higher insulin levels in the blood.
- Glucose dysregulation: Chronic stress can disrupt the normal balance between insulin and glucagon, leading to dysregulated blood sugar levels. Stress hormones, such as cortisol and epinephrine, increase glucose production in the liver (gluconeogenesis) and decrease glucose utilization in peripheral tissues. This can result in elevated blood glucose levels, especially in individuals who are already predisposed to insulin resistance or have underlying metabolic conditions like diabetes.
- Increased glucagon secretion: Chronic stress can also cause an increase in glucagon secretion. Glucagon stimulates glycogen breakdown (glycogenolysis) in the liver and promotes gluconeogenesis, leading to an increased release of glucose into the bloodstream. Elevated glucagon levels, along with disrupted insulin signaling, contribute to hyperglycemia (high blood sugar) in stress-related situations.
- Impaired insulin secretion: Chronic stress can negatively impact the function of pancreatic beta cells, which are responsible for producing insulin. Stress hormones can impair insulin secretion and lead to reduced insulin release in response to elevated blood sugar levels. This further contributes to impaired glucose control and the development of insulin resistance.
- Central nervous system effects: Stress also affects the central nervous system, particularly the hypothalamus-pituitary-adrenal (HPA) axis, which controls the release of stress hormones like cortisol. Dysregulation of the HPA axis can influence insulin and glucagon secretion, disrupt glucose metabolism, and contribute to insulin resistance.
It’s important to note that the impact of chronic stress on insulin and glucagon can vary among individuals, and the underlying mechanisms are complex. However, these effects collectively contribute to the dysregulation of blood sugar control and can increase the risk of developing metabolic disorders such as type 2 diabetes. Managing stress through healthy coping strategies, exercise, adequate sleep, and stress reduction techniques can help mitigate these effects and promote better glucose regulation.

Tips to manage cortisol stress to help make more sex hormones?
While cortisol is a stress hormone that plays a crucial role in the body’s stress response, excessive or chronic elevation of cortisol levels can have negative effects on sex hormone production and balance. To help manage cortisol levels and support the production of sex hormones, here are some tips:
- Stress management techniques: Engage in stress-reducing activities such as deep breathing exercises, meditation, yoga, mindfulness practices, or engaging in hobbies and activities that bring you joy. These techniques can help lower cortisol levels and promote a more balanced hormonal environment.
- Regular exercise: Engaging in regular physical activity, such as aerobic exercises, strength training, or even brisk walking, can help reduce stress and promote the production of endorphins, which are natural mood-enhancing chemicals. Exercise also supports hormonal balance and can positively influence sex hormone production.
- Adequate sleep: Prioritize good sleep hygiene and aim for 7-9 hours of quality sleep each night. Sufficient sleep helps regulate cortisol levels and supports the healthy production of sex hormones.
- Balanced nutrition: Eat a balanced diet that includes a variety of whole foods, including fruits, vegetables, lean proteins, healthy fats, and complex carbohydrates. Ensure you’re getting adequate nutrients like vitamin C, B vitamins, magnesium, and zinc, which are essential for hormone production and regulation.
- Manage caffeine and alcohol intake: Excessive consumption of caffeine and alcohol can disrupt hormone balance and contribute to increased cortisol levels. Moderation is key, so consider limiting or avoiding these substances, especially if you notice they impact your stress levels or sleep quality.
- Supportive supplements: Certain supplements may help support hormone balance and reduce stress. Adaptogenic herbs like ashwagandha and rhodiola rosea can help modulate cortisol levels and support the body’s response to stress. However, it’s important to consult with a healthcare professional before starting any new supplements.
- Social support and relaxation: Cultivate a strong support network of friends, family, or a therapist to help manage stress and provide emotional support. Engaging in activities that promote relaxation, such as taking baths, practicing self-care, or spending time in nature, can also be beneficial for hormonal balance.
Remember, managing cortisol levels and promoting hormone balance is a holistic approach that involves multiple lifestyle factors. It’s important to listen to your body, prioritize self-care, and consult with a healthcare professional for personalized advice based on your specific needs and circumstances.
What is ancestral health and primal diet?
Ancestral Health: Ancestral health, also known as evolutionary health or paleoanthropology, is an interdisciplinary approach that explores how human biology and physiology have been shaped by the environments and lifestyles of our ancient ancestors. It seeks to apply this knowledge to promote optimal health in the modern world.
The central premise of ancestral health is that our genetic makeup and physiology are best adapted to the lifestyles and dietary patterns of our hunter-gatherer ancestors, who lived for the majority of human evolutionary history. Proponents of ancestral health argue that by aligning our behaviors, including diet and physical activity, with our evolutionary heritage, we can enhance our overall well-being and reduce the risk of chronic diseases.
Ancestral health principles often emphasize the following:
- Whole, unprocessed foods: The focus is on consuming minimally processed, nutrient-dense foods, such as lean meats, fish, fruits, vegetables, nuts, seeds, and healthy fats.
- Elimination of processed foods: Highly processed foods, including refined grains, added sugars, and artificial ingredients, are minimized or avoided.
- Physical activity: Regular exercise and physical activity are encouraged, aiming to mimic the active lifestyles of our ancestors.
- Sleep and stress management: Prioritizing quality sleep and stress reduction techniques are considered important factors for overall health.
Primal Diet: The primal diet, also known as the primal blueprint or primal/paleo diet, is a specific dietary approach within the ancestral health framework. It is inspired by the dietary habits of our early human ancestors and focuses on consuming whole, unprocessed foods while eliminating or minimizing modern processed foods.
The primal diet typically includes:
- High-quality animal proteins: Emphasis is placed on consuming grass-fed meats, pastured poultry and eggs, and wild-caught fish, as they are believed to closely resemble the animal proteins consumed by early humans.
- Fresh fruits and vegetables: A variety of non-starchy vegetables and fruits are encouraged for their fiber, phytonutrients, and vitamin content.
- Healthy fats: Primal diets often prioritize healthy fats from sources such as avocados, nuts, seeds, coconut oil, and olive oil.
- Limited grains and legumes: Grains and legumes are generally minimized or eliminated due to their potentially inflammatory properties and antinutrient content.
The primal diet aims to promote overall health, weight management, and blood sugar regulation by emphasizing nutrient-dense whole foods and reducing reliance on processed and refined foods.
It’s important to note that while ancestral health and the primal diet draw insights from our ancestral past, they are not strict historical reenactments. Instead, they provide a framework for making informed dietary and lifestyle choices that support optimal health in the modern context. Individual variations, preferences, and health considerations should be taken into account when adopting any specific dietary approach.
Why eat BUTTER?
Butter, when consumed in moderation as part of a balanced diet, can provide several nutritional benefits.
Here are some of the key nutritional components and benefits of butter:
- Vitamins: Butter is a good source of fat-soluble vitamins, particularly vitamin A, vitamin E, and vitamin K2. Vitamin A is important for vision, immune function, and skin health. Vitamin E is an antioxidant that helps protect cells from oxidative damage, and vitamin K2 plays a role in bone health and blood clotting regulation.
- Healthy fats: Butter contains a mix of saturated, monounsaturated, and polyunsaturated fats. While butter is primarily known for its saturated fat content, it also contains beneficial fatty acids such as butyric acid, a short-chain fatty acid with potential anti-inflammatory properties. Additionally, grass-fed butter can have a higher content of omega-3 fatty acids and conjugated linoleic acid (CLA), which may have various health benefits.
- Conjugated linoleic acid (CLA): CLA is a type of fatty acid found in butter, particularly in the butter from grass-fed cows. CLA has been associated with potential health benefits, including improved body composition, reduced inflammation, and enhanced immune function. However, the levels of CLA can vary depending on factors such as cow diet and breed.
- Fat-soluble activators: Butter contains small amounts of fat-soluble activators, which are compounds that help the body absorb and utilize minerals. These activators include vitamins A, D, and K2, which support the absorption and utilization of minerals such as calcium, phosphorus, and magnesium.
- Flavor and satisfaction: Butter adds flavor and richness to food, enhancing the taste and satisfaction of meals. Including moderate amounts of butter in the diet can make foods more enjoyable, potentially leading to greater overall satisfaction and adherence to a healthy eating plan.
How does grass fed butter help with SCFA production of brutyate?
- Higher omega-3 fatty acid content: Grass-fed butter tends to have a higher content of omega-3 fatty acids, particularly alpha-linolenic acid (ALA). Omega-3 fatty acids can have anti-inflammatory effects and provide the building blocks for the production of butyrate.
- Rich in conjugated linoleic acid (CLA): Grass-fed butter is also a source of conjugated linoleic acid (CLA), a type of fatty acid that has been associated with various health benefits. CLA has been shown to have anti-inflammatory properties and may support the growth of beneficial gut bacteria that produce butyrate.
- Butyrate precursors: Grass-fed butter contains certain nutrients and compounds that can serve as precursors for butyrate production. For example, grass-fed butter is a source of dietary fiber, which can be fermented by gut bacteria to produce SCFAs like butyrate.
- Enhancing gut microbiota diversity: The consumption of grass-fed butter, along with a diverse range of whole foods, can promote a more diverse and balanced gut microbiota. A healthy and diverse gut microbiota is crucial for the production of butyrate by the resident bacteria in the colon.
Butyrate is an important SCFA with numerous benefits for gut health. It serves as the primary energy source for the cells lining the colon, helps maintain gut barrier integrity, reduces inflammation, and supports the overall health of the digestive system.
While grass-fed butter can contribute to butyrate production, it’s worth noting that the overall composition of an individual’s diet and their gut microbiota play significant roles in SCFA production. Consuming a varied diet rich in fiber, including fruits, vegetables, whole grains, and legumes, along with other sources of healthy fats, can further support the production of butyrate and other beneficial SCFAs in the gut.
What are anti nutrients?
How do they impact absorption?
- Phytates: Phytates, also known as phytic acid or inositol hexaphosphate, are found in seeds, nuts, grains, and legumes. They can bind to minerals such as iron, zinc, calcium, and magnesium, forming complexes that are poorly absorbed by the body. This can reduce the bioavailability of these minerals, potentially leading to mineral deficiencies if a diet heavily reliant on phytate-rich foods is consumed.
- Oxalates: Oxalates are found in various plant foods, including spinach, rhubarb, beets, and certain nuts. They can form insoluble crystals with calcium, leading to the formation of kidney stones in susceptible individuals. Individuals with a history of kidney stones or specific medical conditions may need to limit their intake of high-oxalate foods.
- Lectins: Lectins are proteins found in many plant foods, including legumes, grains, and certain vegetables. They have the ability to bind to carbohydrates on the surface of cells, which can interfere with the absorption of nutrients and potentially cause digestive disturbances. Some lectins are more problematic than others, and cooking, soaking, and fermenting can help reduce their levels and mitigate their effects.
- Protease inhibitors: Protease inhibitors are compounds that interfere with the activity of digestive enzymes called proteases, which break down proteins. They are found in foods like legumes (e.g., soybeans) and certain grains. Protease inhibitors may reduce protein digestibility, although the impact is typically minimal in a balanced diet.
- Tannins: Tannins are a group of compounds found in foods such as tea, coffee, fruits (e.g., pomegranates), and certain nuts. They can bind to proteins and minerals, potentially interfering with protein digestion and nutrient absorption. However, moderate consumption of tannin-containing foods is generally considered safe.
It’s important to note that antinutrients are not inherently harmful and can be part of a healthy diet. In fact, many plant foods rich in antinutrients also provide valuable nutrients, fiber, and phytochemicals. The effects of antinutrients can be mitigated through various preparation methods, such as soaking, fermenting, cooking, and combining foods. Additionally, having a varied and balanced diet helps ensure nutrient adequacy and minimizes the potential negative impact of antinutrients.
What are the ANTI-NUTRIENTS in your diet?

How are sex hormones made from high fat foods?
- Cholesterol as a precursor: Cholesterol is a fundamental component in the synthesis of sex hormones. It serves as the starting material from which sex hormones are produced. When cholesterol levels are sufficient, the body can convert it into sex hormones through a series of enzymatic reactions.
- Essential fatty acids: High-fat foods, particularly those rich in healthy fats like monounsaturated fats and omega-3 fatty acids, can support the production of sex hormones indirectly. Essential fatty acids, such as omega-3 and omega-6 fatty acids, are necessary for the production of prostaglandins, which are hormone-like substances involved in regulating various physiological processes, including the reproductive system.
- Hormonal balance: Consuming a well-balanced diet that includes adequate healthy fats can help maintain optimal hormonal balance. Hormones work in delicate interplay, and imbalances can affect the production and regulation of sex hormones. Including healthy fats in the diet can help support the overall hormonal milieu and contribute to the production of sex hormones in appropriate proportions.
It’s important to note that while dietary fats can support the production of sex hormones, it’s a complex process that involves various factors beyond just fat intake. Hormone synthesis and balance are influenced by a range of factors, including overall nutrient intake, lifestyle factors, genetics, and individual health status. Additionally, other nutrients, such as vitamins and minerals, play important roles in hormone production and regulation.
How do you find the fat oxidation rate and fat calories burned for athletes?
Determining the fat oxidation rate and fat calories burned during exercise for athletes typically involves conducting metabolic testing, such as indirect calorimetry or fat oxidation tests. These tests provide valuable insights into an individual’s energy metabolism and can help assess the utilization of fats as a fuel source during exercise. Here’s an overview of the two primary methods used for assessing fat oxidation:
- Indirect Calorimetry: Indirect calorimetry measures the body’s oxygen consumption (VO2) and carbon dioxide production (VCO2) during exercise to estimate energy expenditure and substrate utilization. By analyzing the respiratory gas exchange, it’s possible to determine the relative contribution of fats and carbohydrates to energy production. Fat oxidation rate and fat calories burned can be calculated based on these measurements. Specialized metabolic carts or portable devices are typically used in a lab or clinical setting to collect breath samples and obtain accurate measurements.
- Fat Oxidation Tests: Fat oxidation tests involve administering a substrate, such as a specific fatty acid, labeled with stable isotopes. These isotopes can be traced and measured in exhaled breath or urine samples to quantify the oxidation of fats during exercise. This method provides more precise information on the actual rate of fat oxidation during a specific activity. Fat oxidation tests are often performed in research or specialized sports performance centers.
It’s important to note that these tests require specialized equipment and expertise to conduct accurately. They are typically performed under controlled conditions to obtain reliable data. For athletes seeking to assess their fat oxidation rate or fat calories burned, it’s recommended to consult with sports scientists, exercise physiologists, or sports performance experts who have experience in metabolic testing. These professionals can guide athletes through the testing process, interpret the results, and provide personalized recommendations to optimize energy metabolism during training and competition.
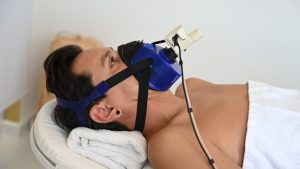
Determining the fat oxidation rate and fat calories burned during exercise for athletes typically involves conducting metabolic testing, such as indirect calorimetry or fat oxidation tests. These tests provide valuable insights into an individual’s energy metabolism and can help assess the utilization of fats as a fuel source during exercise. Here’s an overview of the two primary methods used for assessing fat oxidation:
- Indirect Calorimetry: Indirect calorimetry measures the body’s oxygen consumption (VO2) and carbon dioxide production (VCO2) during exercise to estimate energy expenditure and substrate utilization. By analyzing the respiratory gas exchange, it’s possible to determine the relative contribution of fats and carbohydrates to energy production. Fat oxidation rate and fat calories burned can be calculated based on these measurements. Specialized metabolic carts or portable devices are typically used in a lab or clinical setting to collect breath samples and obtain accurate measurements.
- Fat Oxidation Tests: Fat oxidation tests involve administering a substrate, such as a specific fatty acid, labeled with stable isotopes. These isotopes can be traced and measured in exhaled breath or urine samples to quantify the oxidation of fats during exercise. This method provides more precise information on the actual rate of fat oxidation during a specific activity. Fat oxidation tests are often performed in research or specialized sports performance centers.
It’s important to note that these tests require specialized equipment and expertise to conduct accurately. They are typically performed under controlled conditions to obtain reliable data. For athletes seeking to assess their fat oxidation rate or fat calories burned, it’s recommended to consult with sports scientists, exercise physiologists, or sports performance experts who have experience in metabolic testing. These professionals can guide athletes through the testing process, interpret the results, and provide personalized recommendations to optimize energy metabolism during training and competition.
How does PNOE Metabolic Testing find the fat oxidation rates per minute during exercise?
- Gas Exchange Analysis: PNOE Metabolic Testing involves the measurement of respiratory gases, specifically oxygen consumption (VO2) and carbon dioxide production (VCO2), during exercise. This is typically done using a portable device that captures breath samples.
- Calculation of Respiratory Exchange Ratio (RER): The respiratory exchange ratio (RER) is the ratio of carbon dioxide production to oxygen consumption (VCO2/VO2). It provides information about the proportion of energy derived from carbohydrates versus fats. An RER of 1.0 indicates that carbohydrates are the primary fuel source, while an RER of 0.7 indicates that fats are the primary fuel source. Intermediate values represent a mixture of carbohydrate and fat utilization.
- Fat Oxidation Rate Calculation: Using the measured VO2 and VCO2 values and the RER, PNOE Metabolic Testing can estimate the rate of fat oxidation. The fat oxidation rate is calculated based on the difference between the total energy expenditure and the energy derived from carbohydrates, with the assumption that the remaining energy comes from fat oxidation.
By analyzing the respiratory gas exchange and applying specific equations and algorithms, PNOE Metabolic Testing can provide real-time information on an individual’s fat oxidation rates during exercise. This can be used to determine the rate of fat utilization and help optimize training and nutrition strategies to enhance fat metabolism and endurance performance.
It’s important to note that PNOE Metabolic Testing, like any metabolic testing method, provides estimates and is subject to certain limitations. Factors such as individual variability, exercise intensity, duration, and other physiological factors can affect fat oxidation rates. Interpreting the results and applying them to specific training and nutritional recommendations should be done in consultation with sports scientists, exercise physiologists, or qualified professionals experienced in metabolic testing.What is fat oxidation rate?
ChatGPT
During exercise, the body uses a combination of carbohydrates and fats to meet its energy demands. The intensity and duration of the activity play a role in determining the proportion of energy derived from fats versus carbohydrates. When the intensity is low to moderate and the duration is longer, such as during low-intensity endurance activities, fat oxidation tends to be higher as a percentage of total energy expended.
The fat oxidation rate can be expressed in various ways, including:
- Absolute rate: This represents the amount of fat oxidized per unit of time, such as grams of fat per minute or grams of fat per hour.
- Relative rate: This refers to the proportion of fat oxidation compared to total energy expenditure during a given activity. It is often expressed as a percentage, such as the percentage of energy derived from fat.
Understanding fat oxidation rates can be valuable for individuals interested in optimizing fat metabolism, endurance performance, and body composition goals. Factors that can influence fat oxidation rates include individual metabolic characteristics, exercise intensity, training status, and nutrition.
It’s important to note that the fat oxidation rate is just one aspect of energy metabolism and does not necessarily correlate directly with weight loss or body fat reduction. Achieving a negative energy balance through a combination of energy expenditure (exercise) and energy intake (nutrition) is crucial for fat loss.
Consulting with a healthcare professional, registered dietitian, or exercise physiologist can provide personalized guidance on optimizing fat oxidation rates based on individual goals, health status, and training needs.

