Liver Detoxification Pathways
Purpose of the Liver
The liver is a vital organ that performs a wide range of essential functions, including: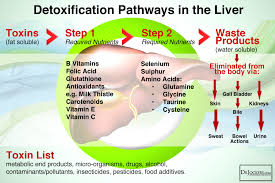
- Detoxification: The liver detoxifies various metabolites, drugs, and toxins, making them less harmful and easier to excrete.
- Metabolism: It plays a central role in carbohydrate, protein, and fat metabolism. This includes the regulation of blood sugar levels, synthesis of cholesterol, and production of bile acids.
- Storage: The liver stores vitamins (A, D, E, K, and B12), minerals (iron and copper), and glycogen (a form of stored glucose).
- Synthesis: It synthesizes essential proteins, such as albumin, clotting factors, and certain hormones.
- Immune Function: The liver contains Kupffer cells, which are part of the immune system and help to destroy pathogens and remove debris from the blood.
- Bile Production: It produces bile, which is essential for the digestion and absorption of fats and fat-soluble vitamins in the small intestine.
Liver Detoxification Pathways
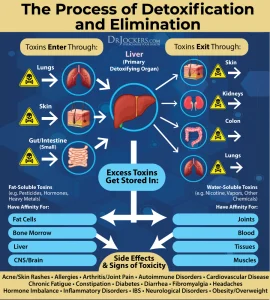
The liver detoxifies harmful substances through a three-phase process: Phase I, Phase II, and Phase III. Each phase is crucial for transforming lipophilic (fat-soluble) toxins into more hydrophilic (water-soluble) forms that can be excreted from the body.
Phase I: Oxidation, Reduction, and Hydrolysis
Purpose:
- The primary purpose of Phase I is to introduce or expose functional groups on toxins to increase their reactivity. This process is often mediated by the cytochrome P450 enzyme family.
- These reactions can create reactive intermediates, including free radicals, which may be more toxic than the original substances.
Reactions:
- Oxidation: Adding oxygen or removing hydrogen atoms (e.g., hydroxylation).
- Reduction: Adding electrons to a substance (e.g., reduction of nitro compounds).
- Hydrolysis: Breaking chemical bonds by adding water (e.g., ester and amide hydrolysis).
Significance:
- The products of Phase I reactions often require further processing in Phase II to become less harmful and more easily excretable.
Phase II: Conjugation
Purpose:
- The primary purpose of Phase II is to conjugate, or link, the reactive intermediates from Phase I with hydrophilic (water-soluble) molecules. This process generally neutralizes the toxicity of the substances and prepares them for excretion.
Pathways:
- Glucuronidation: Adds glucuronic acid to substances.
- Sulfation: Adds sulfate groups.
- Glutathione Conjugation: Adds glutathione.
- Amino Acid Conjugation: Adds amino acids like glycine.
- Acetylation: Adds acetyl groups.
- Methylation: Adds methyl groups.
Significance:
- Conjugated toxins are usually less reactive and more water-soluble, making them easier to excrete via bile or urine.
Phase III: Excretion
Purpose:
- The primary purpose of Phase III is to transport the conjugated toxins out of liver cells and into the bile or bloodstream for elimination from the body.
Mechanisms:
- Transport Proteins: ATP-binding cassette (ABC) transporters, such as multidrug resistance proteins (MRPs) and P-glycoprotein, facilitate the movement of conjugated toxins into bile for excretion via the intestines or into the blood for excretion by the kidneys.
Significance:
- Efficient excretion prevents the accumulation of toxins in the body, protecting tissues and organs from potential damage.
Summary
- Detoxification: The liver detoxifies harmful substances to protect the body from potential damage.
- Phase I: Introduces reactive groups to toxins via oxidation, reduction, and hydrolysis. This phase requires enzymes and produces reactive intermediates.
- Phase II: Conjugates reactive intermediates with hydrophilic molecules, neutralizing their toxicity and making them water-soluble. This phase involves various conjugation pathways and requires specific nutrients and cofactors.
- Phase III: Transports conjugated toxins out of liver cells for excretion via bile or urine, ensuring their removal from the body.
These liver detox pathways work together to convert lipophilic toxins into less harmful, water-soluble forms that can be efficiently excreted, maintaining overall health and reducing oxidative stress.
The Role of the Liver and Detox Pathways in Oxidative Stress
Liver’s Role:
- Detoxification:
- Phase I Reactions (Oxidation, Reduction, Hydrolysis): The liver uses enzymes, primarily from the cytochrome P450 family, to transform lipophilic toxins into more hydrophilic substances. This process often generates free radicals as byproducts, contributing to oxidative stress.
- Phase II Reactions (Conjugation): In this phase, the liver attaches these transformed substances to molecules like glutathione, sulfate, or glucuronic acid to make them water-soluble for easier excretion. Glutathione, a potent antioxidant, helps neutralize free radicals.
- Antioxidant Production:
- Glutathione: The liver synthesizes and regenerates glutathione, one of the body’s most important antioxidants. Glutathione neutralizes free radicals and reactive oxygen species (ROS), helping to protect cells from oxidative damage.
- Nutrient Metabolism:
- Vitamin Storage and Activation: The liver stores and activates vitamins like A, D, E, and K, which have antioxidant properties. It also plays a role in the metabolism of iron and copper, whose excess can catalyze the formation of free radicals.
Detox Pathways:
- Antioxidant Enzymes:
- Superoxide Dismutase (SOD): Converts superoxide radicals into hydrogen peroxide.
- Catalase: Converts hydrogen peroxide into water and oxygen, reducing oxidative stress.
- Glutathione Peroxidase: Uses glutathione to reduce hydrogen peroxide to water.
- Phase III Reactions (Excretion):
- Transporters: The liver uses specific transporters to excrete the conjugated toxins into bile for elimination via the digestive tract or into blood for excretion by the kidneys.
The Role of Mitochondria in Oxidative Stress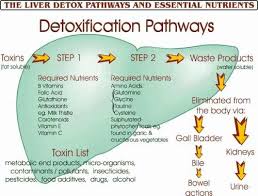
Mitochondria’s Role:
- Energy Production:
- Electron Transport Chain (ETC): Mitochondria are the primary site of cellular respiration, where oxygen is used to produce ATP. During this process, electrons are transferred through the ETC, and a small percentage can leak out and react with oxygen to form superoxide radicals, a type of ROS.
- Free Radical Generation:
- ROS Production: As byproducts of ATP production, mitochondria continuously produce ROS. Under normal conditions, these ROS are kept in check by the cell’s antioxidant defenses. However, during stress or dysfunction, ROS production can increase significantly.
- Regulation of Apoptosis:
- Cytochrome c Release: When oxidative stress damages mitochondria, they can release cytochrome c into the cytoplasm, triggering programmed cell death (apoptosis). This process is crucial for removing damaged cells but can contribute to tissue damage if dysregulated.
Antioxidant Defense in Mitochondria:
- Mitochondrial Antioxidants:
- Manganese Superoxide Dismutase (MnSOD): Converts superoxide radicals produced in the mitochondria into hydrogen peroxide.
- Glutathione: Present in mitochondria to neutralize ROS.
- Thioredoxin and Peroxiredoxin: Enzyme systems within mitochondria that help reduce hydrogen peroxide and other peroxides.
Summary
- Liver and Detox Pathways: The liver plays a critical role in detoxifying harmful substances and neutralizing free radicals. It produces and regenerates antioxidants like glutathione, processes nutrients that have antioxidant properties, and uses enzyme systems to transform and excrete toxins, mitigating oxidative stress.
- Mitochondria: Mitochondria are central to energy production and are a primary source of ROS. They have intrinsic antioxidant systems to manage ROS and prevent oxidative damage. However, mitochondrial dysfunction can lead to increased ROS production and oxidative stress, contributing to cellular damage and aging.
Together, the liver and mitochondria work to balance free radical production and antioxidant defenses, protecting the body from oxidative stress and maintaining cellular health.

Liver Detoxification Pathways
The liver detoxifies harmful substances through three phases: Phase I, Phase II, and Phase III. Each phase involves specific biochemical reactions and requires various nutrients and cofactors to function efficiently.
Phase I: Oxidation, Reduction, and Hydrolysis
Process:
- Phase I involves the introduction of functional groups to lipophilic (fat-soluble) toxins to make them more reactive. This is achieved primarily through the cytochrome P450 enzyme system.
- These reactions can produce reactive intermediates, including free radicals.
Reactions:
- Oxidation: Addition of oxygen or removal of hydrogen (e.g., by cytochrome P450 enzymes).
- Reduction: Addition of electrons (e.g., reduction of nitro groups).
- Hydrolysis: Addition of water to break chemical bonds (e.g., esterases).
Nutrients and Cofactors Needed:
- B Vitamins: Particularly B2 (riboflavin), B3 (niacin), B6 (pyridoxine), B9 (folic acid), and B12 (cobalamin) for cytochrome P450 activity.
- Antioxidants: Vitamin C and E to neutralize free radicals produced.
- Minerals: Iron, magnesium, selenium, and zinc for enzyme function.
- Flavonoids: Found in fruits and vegetables to support enzyme activity.
Phase II: Conjugation
Process:
- Phase II involves conjugation, where reactive intermediates from Phase I are linked with water-soluble molecules to make them easier to excrete.
- This phase reduces the reactivity and toxicity of Phase I intermediates.
Pathways:
- Glucuronidation: Adding glucuronic acid (e.g., by UDP-glucuronosyltransferase).
- Sulfation: Adding sulfate groups (e.g., by sulfotransferase).
- Glutathione Conjugation: Adding glutathione (e.g., by glutathione S-transferase).
- Amino Acid Conjugation: Adding amino acids like glycine (e.g., by amino acid transferases).
- Acetylation: Adding acetyl groups (e.g., by N-acetyltransferase).
- Methylation: Adding methyl groups (e.g., by methyltransferase).
Nutrients and Cofactors Needed:
- Glutathione Precursors: Cysteine, glycine, and glutamine.
- Sulfur-Containing Foods: Garlic, onions, cruciferous vegetables for sulfation.
- Amino Acids: Glycine, taurine, glutamine, methionine for amino acid conjugation.
- Methyl Donors: Methionine, choline, folate, B12 for methylation.
- Co-factors: Magnesium and molybdenum for enzyme activity.

Phase III: Excretion
Process:
- Phase III involves the transport of conjugated toxins out of the cells and into bile or urine for excretion.
- This phase ensures that detoxified substances are removed from the body efficiently.
Pathways:
- Transport Proteins: ATP-binding cassette (ABC) transporters, such as multidrug resistance proteins (MRPs) and P-glycoprotein, facilitate the movement of conjugated toxins.
Nutrients and Cofactors Needed:
- Fiber: Supports the excretion of bile and conjugated toxins through the intestines.
- Water: Essential for kidney function and urinary excretion.
- Electrolytes: Sodium, potassium, and chloride for maintaining hydration and supporting kidney function.
Summary
- Phase I (Oxidation, Reduction, Hydrolysis): Introduces reactive groups to toxins. Requires B vitamins, antioxidants, and minerals.
- Phase II (Conjugation): Links reactive toxins with water-soluble molecules for excretion. Requires amino acids, sulfur-containing foods, methyl donors, and co-factors like magnesium and molybdenum.
- Phase III (Excretion): Transports conjugated toxins out of cells for elimination via bile or urine. Requires fiber, water, and electrolytes.
Ensuring an adequate intake of these nutrients and cofactors is crucial for the effective functioning of liver detox pathways and the management of oxidative stress


