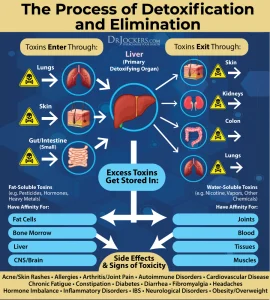
How does CHRONIC Stress impact the WHOLE you?
- Immune system: Chronic stress can suppress the immune system, making individuals more susceptible to infections, illnesses, and autoimmune disorders. Prolonged stress can decrease the production of immune cells, such as lymphocytes, impairing the body’s ability to fight off pathogens effectively.
- Gut health and microbiome: Stress can disrupt the balance of bacteria in the gut, known as the microbiome. This imbalance may lead to digestive issues, inflammation, and increased permeability of the gut lining (leaky gut syndrome), which can contribute to a variety of health problems.
- Brain health: Chronic stress can affect the structure and function of the brain. It can lead to the excessive production of stress hormones, such as cortisol, which can damage brain cells and impair memory, concentration, and mood regulation. It has also been associated with an increased risk of developing mental health disorders like anxiety and depression.
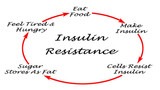
- Insulin and blood glucose: Prolonged stress can influence insulin sensitivity and disrupt the regulation of blood glucose levels. Stress hormones can increase blood sugar levels, which, over time, may contribute to the development of insulin resistance and an increased risk of type 2 diabetes.
- Weight gain: Chronic stress has been linked to weight gain and difficulties in weight management. Stress hormones, such as cortisol, can increase appetite, especially for high-calorie and sugary foods. Additionally, stress can disrupt sleep patterns and lead to emotional eating, both of which can contribute to weight gain.
- Mitochondria: Mitochondria are the energy-producing organelles within cells. Chronic stress can negatively affect mitochondrial function, leading to decreased energy production and an increased risk of fatigue and cellular dysfunction.
- Lean body mass: Prolonged stress can lead to muscle wasting and a decrease in lean body mass. Stress hormones can promote the breakdown of muscle tissue, impairing muscle growth and repair.
It’s important to note that the effects of chronic stress can vary among individuals, and the severity of these impacts can depend on various factors such as genetics, lifestyle, and overall resilience. Managing stress through techniques like regular exercise, adequate sleep, relaxation techniques (e.g., meditation, deep breathing), social support, and engaging in activities you enjoy can help mitigate some of these negative effects and promote better overall health. If you’re experiencing chronic stress and its impacts, it’s advisable to consult with a healthcare professional for personalized guidance and support.
How does CHRONIC Stress impact your Microbiome?
Chronic stress can have a significant impact on the composition and diversity of the microbiome, which refers to the trillions of microorganisms (bacteria, viruses, fungi) residing in our gut. Here’s how chronic stress can affect the microbiome:
- Dysbiosis: Chronic stress can disrupt the balance of the gut microbiome, leading to a condition called dysbiosis. Dysbiosis refers to an imbalance in the types and proportions of bacteria in the gut, with an overgrowth of harmful bacteria and a decrease in beneficial bacteria. This imbalance can affect the microbiome’s overall function and compromise its ability to support digestion, nutrient absorption, and immune regulation.
- Increased permeability: Stress can contribute to increased gut permeability, also known as leaky gut syndrome. Stress hormones like cortisol can cause the tight junctions between the cells lining the intestinal wall to loosen, allowing substances that would normally be restricted to pass through. This can lead to the entry of toxins, bacteria, and other harmful substances into the bloodstream, triggering inflammation and immune responses.
- Inflammation: Chronic stress can induce low-grade inflammation throughout the body, including the gut. Inflammation in the gut can disrupt the balance of the microbiome and compromise its ability to maintain a healthy environment. This can further perpetuate inflammation and lead to a cycle of gut dysfunction.
- Altered microbial metabolites: The gut microbiome plays a crucial role in metabolizing dietary compounds into various metabolites that can have systemic effects. Chronic stress can alter the production and composition of these metabolites, affecting the communication between the microbiome and the host. This disruption can impact various physiological processes and contribute to the development of health problems.
- Immune dysregulation: The gut microbiome plays a vital role in training and modulating the immune system. Chronic stress can disrupt this balance, impairing immune function and compromising the body’s ability to respond appropriately to pathogens and maintain immune tolerance. This can lead to an increased risk of infections, autoimmune disorders, and other immune-related conditions.
It’s important to note that the relationship between chronic stress and the microbiome is complex, and research in this area is ongoing. The specific mechanisms by which stress affects the microbiome are not fully understood. However, evidence suggests that chronic stress can disrupt the delicate balance of the gut microbiome, potentially leading to a cascade of negative effects on overall health. Strategies to manage stress, such as relaxation techniques, regular exercise, and a healthy diet, may help support a healthy microbiome.
How about Pathogens?
Chronic stress can be associated with an increased susceptibility to pathogens due to its impact on the immune system and overall health. Here are some key connections between chronic stress and increased vulnerability to pathogens:
- Immune system suppression: Chronic stress can lead to a suppression of the immune system, impairing its ability to effectively fight off pathogens. Stress hormones like cortisol can reduce the production and function of immune cells, such as lymphocytes and macrophages, which are crucial for identifying and destroying pathogens. This weakened immune response can make individuals more susceptible to infections.
- Inflammation and immune dysregulation: Chronic stress can contribute to chronic low-grade inflammation in the body. Prolonged inflammation can disrupt immune regulation and compromise the body’s defense mechanisms against pathogens. Inflammatory processes can create an environment that favors the growth and survival of pathogens, making it easier for them to establish infections.
- Altered gut microbiota: Chronic stress can disrupt the balance of the gut microbiota, which plays a critical role in immune function. The gut microbiota helps protect against pathogens by competing for resources and producing antimicrobial substances. Stress-induced changes in the gut microbiota can reduce its diversity and alter its composition, weakening the immune defenses against pathogens.
- Impaired barrier function: Chronic stress can compromise the integrity of physical barriers that protect the body from pathogens, such as the skin and mucous membranes. Stress-related changes in blood flow, hormone levels, and barrier maintenance processes can impair the ability of these barriers to prevent the entry of pathogens into the body.
- Unhealthy behaviors and lifestyle: Chronic stress can lead to unhealthy behaviors and lifestyle choices that increase the risk of exposure to pathogens. For example, individuals under chronic stress may have poor sleep patterns, inadequate nutrition, reduced physical activity, and increased substance use. These behaviors can weaken the immune system, making individuals more susceptible to infections.
How does CHRONIC Stress impact your Mighty Mitochondria?
Chronic stress and adrenal exhaustion can impact mitochondria, the energy-producing organelles within our cells, in several ways:
- Energy production: Chronic stress can disrupt the normal functioning of the adrenal glands, which are responsible for producing stress hormones such as cortisol. When stress becomes chronic and the adrenal glands are constantly activated, it can lead to adrenal exhaustion or adrenal fatigue. Adrenal exhaustion can result in reduced cortisol production, which can impact the body’s ability to respond to stress and regulate energy metabolism. As a consequence, the production of energy within the mitochondria may be compromised, leading to feelings of fatigue and decreased physical and mental stamina.
- Oxidative stress: Chronic stress can increase oxidative stress in the body. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to neutralize them with antioxidants. Excessive oxidative stress can damage various cellular structures, including mitochondria. Mitochondria are particularly vulnerable to oxidative damage due to their high metabolic activity and the production of ROS as natural byproducts of energy production. This oxidative damage to mitochondria can impair their function, leading to reduced energy production and further exacerbating the feeling of fatigue.
- Mitochondrial DNA (mtDNA) damage: Mitochondria have their own DNA called mitochondrial DNA (mtDNA). Chronic stress and the associated oxidative stress can damage mtDNA, leading to mitochondrial dysfunction. When mitochondria are unable to function optimally due to mtDNA damage, it can affect their ability to produce energy efficiently. Moreover, damaged mitochondria can produce more ROS, creating a vicious cycle of oxidative stress and further damage to both mtDNA and the mitochondria themselves.
- Inflammation: Chronic stress can promote systemic inflammation, which can impact mitochondrial function. Inflammatory signals released during chronic stress can interfere with the proper functioning of mitochondria and disrupt their ability to generate energy. In turn, dysfunctional mitochondria can contribute to a perpetuation of inflammation, creating a harmful cycle.
- Metabolic dysfunction: Chronic stress and adrenal exhaustion can disrupt metabolic processes, including insulin regulation and glucose metabolism. Dysfunctional mitochondria can impair the body’s ability to utilize glucose for energy efficiently, leading to higher blood sugar levels and increased insulin resistance. These metabolic disturbances can further impact mitochondrial function and energy production.
It’s important to note that the impact of chronic stress and adrenal exhaustion on mitochondria can vary among individuals, and additional factors like genetics, lifestyle, and overall health status can influence these effects.
Managing stress, adopting healthy lifestyle habits (including adequate rest, nutrition, and exercise), and seeking support from healthcare professionals are essential in mitigating the negative impact of chronic stress on mitochondria and overall energy metabolism.
Chronic Stress & Cell Danger Response Theory
Here’s an overview of their relationship:
- Chronic stress and CDR: Chronic stress can activate the CDR, which is a protective response triggered by cells in response to various threats, including stress, infection, or inflammation. The CDR involves a cascade of cellular and molecular changes aimed at maintaining cellular integrity and promoting survival. However, when the CDR is dysregulated or excessively activated for prolonged periods, it can lead to dysfunction in various cellular processes, including mitochondrial function.
- Mitochondria and chronic fatigue: Mitochondria play a central role in energy production, and disruptions in mitochondrial function have been implicated in the development of chronic fatigue. It is hypothesized that chronic stress, through mechanisms like oxidative stress and inflammation, can impair mitochondrial function and energy metabolism. This mitochondrial dysfunction may contribute to the symptoms of chronic fatigue, including persistent fatigue, reduced exercise tolerance, and impaired recovery from exertion.
- CDR and mitochondrial dysfunction: The dysregulation of the CDR, often associated with chronic stress and other triggers, can impact mitochondrial function. The activation of the CDR can lead to a state of cellular defense and energy conservation, which may result in decreased mitochondrial activity and ATP production. Additionally, the excessive production of reactive oxygen species (ROS) during the CDR can contribute to oxidative damage to mitochondrial components, further impairing mitochondrial function.
- Chronic fatigue syndrome (CFS): CFS is a complex and poorly understood condition characterized by persistent and debilitating fatigue that is not alleviated by rest and is often accompanied by a range of other symptoms. While the exact causes of CFS remain uncertain, it is believed that multiple factors, including genetic, environmental, and immune system dysregulation, may contribute to its development. Chronic stress, dysregulated CDR, and mitochondrial dysfunction are among the proposed mechanisms that may play a role in CFS.
It’s important to note that the relationship between chronic stress, the CDR, mitochondria, and chronic fatigue is still an area of ongoing research. While there is evidence to suggest these connections, more studies are needed to fully understand the underlying mechanisms and their specific roles in the development and perpetuation of chronic fatigue.
What nutrients are needed to support “CDR” and Mitochondrial Health?
Improving cell danger response and mitochondrial health often involves a multifaceted approach that includes a balanced diet providing essential nutrients. While specific nutritional needs may vary among individuals, here are some key nutrients that play important roles in supporting cell danger response and mitochondrial health:
- Coenzyme Q10 (CoQ10): CoQ10 is a vital component of the electron transport chain within mitochondria, facilitating ATP production and acting as an antioxidant. It is found in various foods, particularly organ meats, oily fish, nuts, and seeds. In some cases, CoQ10 supplementation may be considered under the guidance of a healthcare professional.
- B Vitamins: B vitamins, including B1 (thiamine), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folate), and B12 (cobalamin), are essential for energy metabolism and mitochondrial function. These vitamins are found in a range of foods, such as whole grains, legumes, leafy greens, meat, fish, and eggs.
- Magnesium: Magnesium is involved in hundreds of biochemical reactions in the body, including energy production and mitochondrial function. Good food sources of magnesium include leafy green vegetables, nuts, seeds, whole grains, and legumes.
- Omega-3 Fatty Acids: Omega-3 fatty acids, particularly EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid), have been associated with improved mitochondrial function and reduced inflammation. They can be found in fatty fish like salmon, mackerel, and sardines, as well as in chia seeds, flaxseeds, and walnuts.
- Antioxidants: Antioxidants, such as vitamins C and E, selenium, and various phytonutrients (e.g., flavonoids, carotenoids), can help counteract oxidative stress and protect mitochondria from damage. Colorful fruits and vegetables, nuts, seeds, and whole grains are excellent sources of antioxidants.
- L-carnitine: L-carnitine plays a critical role in transporting fatty acids into mitochondria for energy production. Good dietary sources of L-carnitine include meat, poultry, fish, and dairy products.
- Antioxidant Enzymes: The body produces antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase, which help neutralize excess reactive oxygen species (ROS). Consuming foods rich in antioxidants and providing the necessary building blocks for these enzymes, such as zinc, copper, selenium, and manganese, can support their production. Food sources include nuts, seeds, legumes, whole grains, and seafood.
It’s important to note that the best approach to improving cell danger response and mitochondrial health is to follow a balanced and varied diet that provides a wide range of nutrients. Nutritional needs can vary depending on individual factors, health conditions, and specific requirements. If you have concerns about your nutrition or are considering supplementation, it’s advisable to consult with a healthcare professional
What nutrients get depleted with Chronic Stress?
Chronic stress can lead to increased nutrient requirements and potential depletion of certain nutrients. Here are some nutrients that can be affected by chronic stress:
- B Vitamins: Chronic stress can deplete B vitamins, particularly B5 (pantothenic acid) and B6 (pyridoxine). These vitamins are involved in energy production, neurotransmitter synthesis, and hormone regulation. Chronic stress increases the demand for B vitamins, potentially leading to their depletion.
- Vitamin C: Chronic stress can increase the production of stress hormones, which can deplete vitamin C. Vitamin C is a powerful antioxidant that supports the immune system and helps manage stress. During stressful periods, the body’s vitamin C stores may become depleted, requiring additional intake.
- Magnesium: Stress can increase the excretion of magnesium through urine, leading to a deficiency over time. Magnesium plays a crucial role in relaxation, stress management, and the functioning of the nervous system. Chronic stress can deplete magnesium levels, making it important to ensure adequate intake.
- Zinc: Chronic stress can lead to increased zinc excretion and impaired zinc absorption. Zinc is involved in immune function, wound healing, and the regulation of stress hormones. Chronic stress can deplete zinc levels, potentially affecting immune system function and overall health.
- Omega-3 Fatty Acids: Chronic stress can disrupt the balance of omega-3 and omega-6 fatty acids. Stress hormones can promote inflammation, and omega-3 fatty acids have anti-inflammatory properties. During chronic stress, the demand for omega-3 fatty acids may increase, potentially leading to a depletion if the diet lacks sufficient intake.
- Antioxidants: Chronic stress can increase oxidative stress in the body, depleting antioxidants such as vitamin E, selenium, and glutathione. These antioxidants are important for combating free radicals and reducing cellular damage caused by stress. Maintaining adequate intake of antioxidants is crucial to counteract the effects of chronic stress.
It’s important to note that nutrient depletion due to chronic stress can vary among individuals and is influenced by factors such as diet, lifestyle, and overall health status.
How does STRESS impact your microbiome?
Chronic stress can weaken the immune system and make individuals more susceptible to pathogens and parasites for several reasons:
- Suppression of immune function: Chronic stress can suppress immune system activity, particularly the cellular immune response. Stress hormones, such as cortisol, can interfere with the production and function of immune cells, including lymphocytes and natural killer (NK) cells, which play crucial roles in defending against pathogens and parasites. This suppression weakens the body’s ability to mount an effective immune response and increases susceptibility to infections.
- Inflammation and immune dysregulation: Chronic stress can lead to chronic low-grade inflammation in the body. Prolonged inflammation can disrupt immune regulation, impair the balance between pro-inflammatory and anti-inflammatory processes, and compromise immune defense mechanisms. This dysregulation can create an environment that favors the growth and survival of pathogens and parasites.
- Altered gut microbiota: Chronic stress can impact the composition and diversity of the gut microbiota—the collection of microorganisms in the digestive tract. The gut microbiota plays a crucial role in immune function, and disruptions in its balance can weaken immune responses. Stress-induced alterations in the gut microbiota can lead to reduced production of antimicrobial substances and impaired gut barrier function, making individuals more susceptible to infections by pathogens and parasites.
- Impaired wound healing: Chronic stress can delay wound healing, which can create an opportunity for pathogens to invade and establish infections. Stress-induced changes in inflammatory responses, blood flow, and the production of growth factors can hinder the normal healing process, leaving wounds vulnerable to colonization by pathogens.
- Changes in behavior and lifestyle: Chronic stress can lead to unhealthy behaviors and lifestyle choices that further increase the risk of exposure to pathogens and parasites. For example, individuals under chronic stress may have poor sleep patterns, inadequate nutrition, reduced physical activity, and increased substance use, all of which can weaken the immune system and make them more susceptible to infections.
It’s important to manage chronic stress effectively through stress-reduction techniques, lifestyle modifications, and seeking support from healthcare professionals. By addressing chronic stress, individuals can help support their immune system function and reduce their susceptibility to pathogens and parasites.
How does CHRONIC Stress impact your digestion? 
Chronic stress can have significant effects on digestion and disrupt the normal functioning of the gastrointestinal (GI) system. The connection between stress and digestion is often referred to as the “brain-gut axis” or the “gut-brain connection.” Here are some ways in which chronic stress can impact digestion:
- Increased sensitivity and motility: Chronic stress can increase the sensitivity of the GI tract, leading to heightened perception of pain and discomfort. It can also affect the motility of the digestive system, causing either increased or decreased bowel movements. Some individuals may experience more frequent or urgent bowel movements (diarrhea), while others may experience slower bowel movements (constipation).
- Changes in appetite: Stress can disrupt normal appetite regulation, leading to changes in eating patterns. Some people may experience increased appetite and overeating, often preferring high-calorie and comfort foods. Others may experience a decrease in appetite, leading to weight loss or inadequate nutrient intake.
- Altered stomach acid secretion: Stress can influence the production of stomach acid. Some individuals may experience increased acid production, which can lead to acid reflux, heartburn, or stomach ulcers. Others may have reduced acid production, affecting digestion and nutrient absorption.
- Impaired nutrient absorption: Chronic stress can affect the functioning of the small intestine, potentially compromising nutrient absorption. This can lead to deficiencies in essential vitamins, minerals, and other nutrients, even if a person has a well-balanced diet.
- Disruption of gut microbiota: The gut microbiota, the community of microorganisms in the digestive tract, plays a crucial role in digestion and overall health. Chronic stress can disrupt the balance of the gut microbiota, potentially leading to digestive issues, inflammation, and immune dysregulation.
- Increased inflammation: Stress triggers the release of pro-inflammatory substances in the body. Chronic inflammation in the GI tract can contribute to conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and other digestive disorders.
- Functional gastrointestinal disorders: Chronic stress can contribute to the development or exacerbation of functional gastrointestinal disorders (FGIDs) such as IBS. These disorders are characterized by recurring digestive symptoms, including abdominal pain, bloating, and altered bowel habits, in the absence of identifiable structural or biochemical abnormalities.
It’s important to note that the impact of chronic stress on digestion can vary between individuals. Some people may be more susceptible to stress-related digestive issues than others. Managing stress through techniques like relaxation exercises, mindfulness, regular exercise, and seeking support from healthcare professionals or mental health providers can help mitigate the negative effects of chronic stress on digestion.
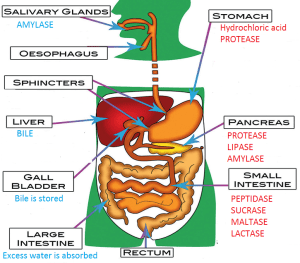
What digestive functions occur in the Parasympathetic Nervous System?
The parasympathetic nervous system plays a vital role in regulating and promoting various digestive functions. It is often referred to as the “rest and digest” system because it promotes a relaxed state conducive to optimal digestion and nutrient absorption.
Here are some key digestive functions controlled by the parasympathetic nervous system:
- Salivation: The parasympathetic system stimulates the salivary glands, leading to increased saliva production. Saliva contains enzymes that initiate the breakdown of carbohydrates, aiding in the process of digestion.
- Gastric secretions: The parasympathetic nervous system stimulates the release of gastric juices in the stomach. These juices, including hydrochloric acid and enzymes like pepsin, help break down food and facilitate the digestion of proteins.
- Motility and peristalsis: Parasympathetic signals enhance the contraction of smooth muscles in the digestive tract, promoting peristalsis—the rhythmic contractions that propel food along the gastrointestinal (GI) tract. This movement aids in the mixing and digestion of food.
- Pancreatic enzyme secretion: The parasympathetic system stimulates the pancreas to secrete digestive enzymes, such as amylase, lipase, and proteases. These enzymes are crucial for the breakdown of carbohydrates, fats, and proteins in the small intestine.
- Gallbladder contraction: The parasympathetic signals trigger the contraction of the gallbladder, which releases bile into the small intestine. Bile aids in the digestion and absorption of fats.
- Intestinal blood flow: The parasympathetic nervous system promotes vasodilation in the blood vessels supplying the intestines. This increased blood flow ensures an adequate supply of oxygen and nutrients to support the digestive processes.
- Sphincter control: Parasympathetic stimulation relaxes the smooth muscles of certain sphincters in the GI tract, allowing for the passage of food and waste materials. For example, it relaxes the lower esophageal sphincter, facilitating the movement of food from the esophagus to the stomach.
Overall, the parasympathetic nervous system facilitates and coordinates the digestive processes by increasing secretions, enhancing motility, and optimizing the function of various digestive organs.
It promotes a state of relaxation and optimal digestion, allowing the body to efficiently break down and absorb nutrients from the food we consume.
How can you shift to Parasympathetic before eating to improve digestion?
Shifting from the sympathetic nervous system dominance to the parasympathetic nervous system can help promote proper digestion and optimize the functioning of the digestive system. Here are some tips to facilitate this shift and promote a relaxed state for digestion:
- Practice deep breathing: Deep, diaphragmatic breathing activates the parasympathetic nervous system and induces a state of relaxation. Take slow, deep breaths, focusing on breathing from your abdomen rather than shallow chest breathing. This can help calm your body and mind, promoting the shift to the parasympathetic state.
- Engage in relaxation techniques: Incorporate relaxation techniques such as meditation, mindfulness, progressive muscle relaxation, or yoga into your daily routine. These practices can help reduce stress levels and activate the parasympathetic system, facilitating optimal digestion.

- Eat mindfully: Take the time to eat your meals in a calm and mindful manner. Avoid rushing or eating on the go. Chew your food thoroughly and savor the flavors. This signals your body that you are in a relaxed state and promotes proper digestion.
- Create a soothing environment: Create a peaceful and calming environment during mealtime. Sit in a comfortable and quiet space, free from distractions like electronic devices or television. Playing soft, soothing music or enjoying a pleasant ambiance can further enhance relaxation.
- Reduce stress levels: Identify and address sources of stress in your life. Engage in stress management techniques that work for you, such as exercise, spending time in nature, engaging in hobbies, or seeking support from loved ones or a mental health professional. By reducing overall stress, you can support the shift towards the parasympathetic state.
- Incorporate physical activity: Regular physical activity, such as moderate exercise or walks, can help promote overall relaxation and balance between the sympathetic and parasympathetic systems. Find activities you enjoy and make them a part of your routine.
- Prioritize self-care: Take care of yourself by getting enough sleep, maintaining a balanced diet, and engaging in activities that bring you joy and relaxation. Self-care practices support overall well-being and contribute to a state of calm conducive to proper digestion.
Remember that everyone’s response to these techniques may vary, and it’s essential to find what works best for you. If you experience chronic stress or digestive issues, it is advisable to consult with a healthcare professional for personalized guidance and support.
Test and not guess on your digestive dysfunction & infections!
Chronic stress has been suggested to potentially impact the risk and severity of H. pylori infection, but the relationship is complex and not fully understood. Here’s an overview of the possible connections between chronic stress and H. pylori infection:
- Impact on immune system: Chronic stress can suppress the immune system and affect its ability to fight off infections effectively. This weakened immune response may potentially make individuals more susceptible to acquiring H. pylori infection or increase the risk of persistent infection.
- Gastric acid secretion: Chronic stress can affect gastric acid secretion. While stress is not a direct cause of H. pylori infection, altered gastric acid levels due to stress may create an environment that is more conducive to the survival and growth of H. pylori bacteria. Higher acid levels can help protect against initial H. pylori colonization, while reduced acid levels may favor its establishment.
- Disease progression: Once H. pylori infection is established, chronic stress may play a role in the progression of related gastrointestinal conditions. Research suggests that stress can exacerbate the symptoms of gastritis, peptic ulcers, and other H. pylori-associated diseases. Stress-induced alterations in gastric acid secretion and immune response can potentially contribute to the development of more severe forms of H. pylori-related conditions.
- Interactions with stress hormones: The interaction between chronic stress and stress hormones like cortisol may have implications for H. pylori infection. Some studies suggest that cortisol, which is released during stress, may influence the growth and virulence of H. pylori bacteria. However, the exact mechanisms and their clinical significance are not yet fully understood.
It’s important to note that the relationship between chronic stress and H. pylori infection is complex and multifactorial. Other factors, such as socioeconomic status, lifestyle habits, and genetic predispositions, also contribute to the risk and outcomes of H. pylori infection. Additionally, H. pylori is primarily transmitted through person-to-person contact, contaminated food, or water, rather than being solely influenced by stress.
If you suspect you have an H. pylori infection or have concerns about stress-related gastrointestinal issues, it is advisable to consult with a healthcare professional. They can provide appropriate testing, diagnosis, and recommend suitable treatment options based on your specific situation.
There is a growing body of research exploring the potential links between H. pylori infection and autoimmune diseases. While the exact mechanisms are not fully understood, some studies have suggested that H. pylori infection may contribute to the development or exacerbation of certain autoimmune conditions.
Here are some key findings:
- Rheumatoid arthritis (RA): Several studies have investigated the association between H. pylori infection and RA. Some research suggests that H. pylori infection may be more prevalent in individuals with RA compared to the general population. However, the results have been conflicting, and further research is needed to establish a clear relationship.
- Immune dysregulation: H. pylori infection has been implicated in promoting immune dysregulation, including the disruption of T-cell balance and the activation of pro-inflammatory immune responses. These immune disturbances may potentially contribute to the development of autoimmune diseases in susceptible individuals.
- Autoimmune gastritis: H. pylori infection is a known risk factor for autoimmune gastritis, a condition characterized by chronic inflammation of the stomach lining and the production of autoantibodies against stomach cells. This condition can lead to the impairment of gastric acid production and vitamin B12 deficiency.
- Systemic lupus erythematosus (SLE): Some studies have suggested a potential association between H. pylori infection and SLE. H. pylori infection may induce an immune response and trigger an autoimmune reaction in genetically susceptible individuals, potentially contributing to the development or exacerbation of SLE.
H. pylori infection can potentially impact protein digestion and amino acid balance, although the exact mechanisms are not fully understood. Here are some ways in which H. pylori infection may affect protein digestion and amino acid balance:
- Gastric acid production: H. pylori infection can lead to chronic inflammation of the stomach lining (gastritis) and damage to the gastric mucosa. In some cases, this can result in reduced gastric acid secretion, which is necessary for the proper digestion of proteins. Inadequate gastric acid levels may impair the activation of pepsin, an enzyme responsible for breaking down proteins into smaller peptides.
- Disruption of digestive enzymes: H. pylori infection may interfere with the production and activity of digestive enzymes in the stomach and small intestine. Digestive enzymes, such as pepsin and pancreatic proteases, are crucial for protein breakdown and the release of amino acids for absorption. Altered enzyme activity due to H. pylori infection may impair protein digestion and nutrient absorption.
- Impact on gastric motility: H. pylori infection can affect gastric motility, the movement of food through the stomach. This can result in delayed gastric emptying, reducing the time available for protein digestion. Slower gastric motility may also lead to prolonged exposure of proteins to the acidic environment of the stomach, potentially affecting their digestion.
- Altered nutrient absorption: H. pylori infection and associated gastritis can affect the overall health and function of the stomach and small intestine, potentially impairing nutrient absorption. Reduced absorption of amino acids from digested proteins can disrupt the balance of amino acids in the body and affect overall amino acid availability for various physiological processes.
- Nutritional deficiencies: In severe cases of H. pylori infection, especially those associated with autoimmune gastritis, the damage to the stomach lining can impair the production of intrinsic factor—a protein necessary for the absorption of vitamin B12. Vitamin B12 deficiency can affect red blood cell production and lead to anemia, which can further impact protein metabolism and overall amino acid balance.
It’s important to note that the effects of H. pylori infection on protein digestion and amino acid balance can vary between individuals and depend on the severity and duration of the infection.
Additionally, the impact may be more significant in cases of chronic or persistent H. pylori infection. If you suspect an H. pylori infection or have concerns about protein digestion and amino acid balance, it is advisable to consult with a healthcare professional who can provide appropriate evaluation, diagnosis, and treatment options based on your specific situation.
The stomach primarily serves as a site for the initial stages of digestion rather than the synthesis of nutrients, minerals, vitamins, and cofactors.
However, it plays a crucial role in the absorption of some nutrients and the activation of certain substances. Here are a few examples:
- Intrinsic factor: The stomach lining produces intrinsic factor, a glycoprotein necessary for the absorption of vitamin B12. Intrinsic factor binds to vitamin B12 in the stomach and facilitates its absorption in the small intestine.
- Hydrochloric acid (HCl): The stomach secretes hydrochloric acid, which helps in the breakdown of proteins and activates the enzyme pepsinogen to its active form, pepsin. Pepsin aids in the digestion of proteins into smaller peptides.
- Gastric enzymes: The stomach produces gastric enzymes such as gastric lipase, which contributes to the initial digestion of dietary fats.
It’s important to note that while the stomach has limited involvement in the production of nutrients, the small intestine is the primary site for the absorption of various nutrients, minerals, vitamins, and cofactors. Once nutrients are broken down in the stomach and further digested in the small intestine, they are absorbed into the bloodstream and transported to different tissues and organs for utilization.
The synthesis of nutrients, minerals, vitamins, and cofactors primarily occurs in other organs and tissues of the body, such as the liver, kidneys, intestines, and various glands. These organs are responsible for the production, storage, and release of specific nutrients and cofactors required for various physiological functions.
The production of stomach acid (hydrochloric acid, or HCl) involves several factors and cofactors that facilitate its secretion.Here are some key cofactors involved in stomach acid production:
- Histamine: Histamine is an important cofactor that stimulates the release of gastric acid. It acts on specific receptors called histamine H2 receptors present on the acid-secreting cells (parietal cells) in the stomach lining. Binding of histamine to these receptors triggers the release of HCl.
- Gastrin: Gastrin is a hormone produced by specialized cells in the stomach called G cells. It acts as a cofactor for acid production by stimulating the release of gastric acid from parietal cells. Gastrin is released in response to various stimuli, including the presence of food in the stomach, stretching of the stomach wall, and the action of certain chemicals.
- Acetylcholine: Acetylcholine is a neurotransmitter that plays a role in stimulating the secretion of gastric acid. It is released from nerve fibers in the stomach and binds to receptors on parietal cells, promoting acid production.
- Carbonic anhydrase: Carbonic anhydrase is an enzyme found in the parietal cells of the stomach lining. It catalyzes the conversion of carbon dioxide and water into carbonic acid, which then dissociates to form bicarbonate ions and hydrogen ions (protons). The hydrogen ions are actively transported into the stomach lumen, contributing to the acidity of gastric acid.
- Chloride ions: Chloride ions (Cl-) play a critical role in the formation of gastric acid. They are actively transported into the stomach lumen by a specific transporter known as the chloride/bicarbonate exchanger. In exchange for chloride ions entering the stomach, bicarbonate ions exit the stomach and enter the bloodstream.
These cofactors work together to regulate the secretion of stomach acid and maintain the acidity required for proper digestion. Dysregulation of these cofactors can lead to imbalances in acid production and may contribute to various digestive disorders, such as acid reflux, gastritis, or gastric ulcers.
It’s important to note that the regulation of stomach acid production is a complex process involving the coordination of various signals, including neural, hormonal, and local factors. The precise interplay of these factors ensures the appropriate production of stomach acid for effective digestion.
Zinc, iron, and B-vitamins are not directly involved in the production of stomach acid (hydrochloric acid, or HCl). However, these nutrients are essential for overall digestive health and can indirectly influence the function of the stomach and the production of stomach acid.
Here’s a brief overview:
- Zinc: Zinc is a mineral that plays a vital role in various aspects of digestion, including the synthesis of enzymes involved in protein digestion. Adequate zinc levels are necessary for the normal functioning of taste and smell receptors, which can indirectly impact the production of stomach acid. Additionally, zinc deficiency may lead to impaired immune function and increased susceptibility to H. pylori infection, which can affect the health of the stomach lining and potentially influence stomach acid production.
- Iron: Iron is crucial for the production of red blood cells and oxygen transport in the body. While it does not directly affect stomach acid production, iron deficiency anemia can lead to symptoms like gastritis and reduced gastric acid secretion. Conversely, excessive iron intake may cause gastrointestinal irritation, which can affect stomach acid balance.
- B-vitamins: B-vitamins, such as vitamin B12, play important roles in the overall health of the gastrointestinal system. Vitamin B12 deficiency, often associated with autoimmune gastritis or inadequate absorption, can lead to pernicious anemia and damage to the stomach lining, potentially affecting the production of stomach acid. Other B-vitamins, such as thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6), and pantothenic acid (B5), are also involved in various metabolic processes and enzyme functions that indirectly support overall digestion and gut health.
While these nutrients are not directly involved in stomach acid production, their deficiencies or imbalances can impact the health and function of the digestive system, potentially affecting the production of stomach acid and overall digestive processes. It’s important to maintain a well-balanced diet that provides adequate amounts of these nutrients for optimal digestive health. If you have concerns about nutrient deficiencies or digestive issues, consulting with a healthcare professional or registered dietitian can provide personalized guidance and appropriate recommendations.
Poor protein digestion can impact muscle growth and repair.
Proteins are the building blocks of muscles, and their adequate digestion and absorption are crucial for muscle protein synthesis, which is the process by which new muscle proteins are created.
When protein digestion is impaired, such as due to insufficient stomach acid, enzyme deficiencies, or gastrointestinal disorders, the body may struggle to break down dietary proteins into their individual amino acids. As a result, the availability of essential amino acids, which are necessary for muscle repair and growth, may be compromised.
Inadequate protein digestion can lead to reduced absorption of amino acids, resulting in decreased availability of the building blocks required for muscle protein synthesis. This can negatively impact muscle repair after exercise-induced damage and limit the potential for muscle growth and adaptation to training.
Furthermore, impaired protein digestion can result in increased protein fermentation by gut bacteria, leading to the production of metabolites like ammonia and various compounds that can have detrimental effects on muscle health and overall performance.
It’s important to ensure optimal protein digestion and absorption for individuals aiming to support muscle growth and repair. This can be achieved by maintaining a healthy digestive system, including adequate stomach acid production, enzyme function, and gut health. Additionally, consuming a well-balanced diet that provides sufficient high-quality protein sources can help support muscle protein synthesis and promote muscle growth and repair.
Improving protein digestion can be beneficial for optimizing nutrient absorption and supporting overall digestive health.
Here are some tips to enhance protein digestion:
- Adequate stomach acid: Sufficient production of stomach acid (hydrochloric acid, or HCl) is essential for protein digestion. Adequate stomach acid levels help activate the enzyme pepsin, which breaks down proteins into smaller peptides.
- To support stomach acid production, you can:
- Avoid overconsumption of antacids or acid-blocking medications unless medically necessary.
- Consider consuming foods that promote natural acid production, such as apple cider vinegar or fermented foods (e.g., sauerkraut).
- Eat protein-rich foods at the beginning of a meal to ensure optimal exposure to stomach acid.
- Digestive enzymes: Digestive enzymes help break down proteins into amino acids, facilitating their absorption.
- Consider the following: Include enzyme-rich foods in your diet, such as pineapple (contains bromelain) or papaya (contains papain)If needed, consider taking digestive enzyme supplements containing proteases, which aid in protein breakdown.
- Consult with a healthcare professional for guidance.
- Optimal gut health: A healthy gut environment promotes proper digestion and nutrient absorption.
- Take the following steps to support gut health:
- Include a variety of fiber-rich foods in your diet, such as fruits, vegetables, and whole grains. Fiber helps promote regular bowel movements and overall gut health.
- Consume fermented foods like yogurt, kefir, sauerkraut, or kimchi, which contain beneficial bacteria (probiotics) that aid in digestion.
- Limit or avoid foods that you may personally find difficult to digest or that cause gastrointestinal discomfort.
- Chewing and mindful eating: Properly chewing your food aids in the mechanical breakdown of proteins and initiates the digestive process.
- Be mindful of your eating habits:
- Chew your food thoroughly before swallowing.
- Avoid eating too quickly or while feeling stressed, as it can impact digestion and nutrient absorption.
- Address digestive disorders: If you suspect underlying digestive disorders or conditions impacting protein digestion, such as low stomach acid or enzyme deficiencies, consult with a healthcare professional for evaluation, diagnosis, and appropriate treatment.
It’s important to note that individual needs and conditions may vary. If you have specific concerns or persistent digestive issues, it is advisable to seek guidance from a healthcare professional or registered dietitian who can provide personalized recommendations based on your situation.