Why is there so much hype on ZONE TWO and ZONE FIVE heart rate training these days?

The Role of our Mighty Mitochondria!
Mitochondria are membrane-bound organelles found in the cells of most organisms.
They are often referred to as the “powerhouses of the cell” because their primary function is to generate energy in the form of adenosine triphosphate (ATP) through oxidative phosphorylation.
Mitochondria play a crucial role in cellular metabolism, and their functions extend beyond energy production to include regulation of cell cycle and cell growth, as well as involvement in signaling pathways and apoptosis.
- Mitochondria Function:
- Definition: Mitochondria function refers to the various biochemical processes that mitochondria perform within a cell. This includes the production of ATP through oxidative phosphorylation, regulation of reactive oxygen species (ROS), and participation in cellular signaling pathways.
- Importance: Mitochondria function is essential for overall cellular health and function. Impaired mitochondrial function is associated with various diseases and the aging process.
- Mitochondria Density:
- Definition: Mitochondria density refers to the number of mitochondria within a given volume or area of a cell or tissue. It is a measure of the abundance of mitochondria in a particular cellular environment.
- Importance: Mitochondria density can impact the overall metabolic capacity of a cell. Tissues with high energy demands, such as muscles and neurons, tend to have higher mitochondria density.
- Improving Mitochondria Function and Density:

- Regular Exercise: Physical activity, especially aerobic exercise, has been shown to enhance mitochondrial function and increase mitochondria density.
- Healthy Diet: Nutrient-rich diets, including antioxidants and essential nutrients, support mitochondrial health. This includes foods rich in omega-3 fatty acids, antioxidants, and vitamins.
- Mitochondrial Biogenesis: Certain lifestyle factors, such as caloric restriction and intermittent fasting, may stimulate mitochondrial biogenesis—the process of generating new mitochondria.
- Supplements: Some supplements, such as coenzyme Q10, L-carnitine, and alpha-lipoic acid, are believed to support mitochondrial function.
- Improving Mitophagy and Mitochondrial Turnover:
- Exercise: Regular physical activity has been linked to increased mitophagy, the process by which damaged or dysfunctional mitochondria are removed from the cell.
- Fasting: Intermittent fasting or calorie restriction may stimulate autophagy, including mitophagy, which helps eliminate damaged mitochondria.
- Pharmacological Interventions: Some drugs and compounds, such as rapamycin and resveratrol, have been studied for their potential to enhance mitophagy and improve mitochondrial turnover.
- Aging and Mitochondrial Health:
- Mitochondrial Decline with Age: Aging is associated with a decline in mitochondrial function and an increase in oxidative stress. This can contribute to age-related diseases and the aging process itself.
- Lifestyle Interventions: Healthy lifestyle choices, including regular exercise, a balanced diet, and stress management, can help mitigate the decline in mitochondrial function associated with aging.
It’s important to note that while these strategies may support mitochondrial health, individual responses can vary, and it’s always advisable to consult with healthcare professionals before making significant changes to lifestyle or incorporating new supplements.
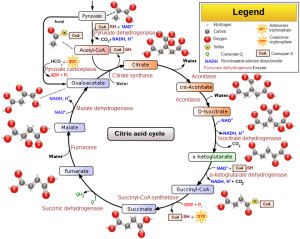
Mitochondria play a central role in several key cellular processes, including oxidative phosphorylation, regulation of reactive oxygen species (ROS), and participation in cellular signaling pathways. Let’s delve into each of these functions:
Oxidative Phosphorylation:
-
- Definition: Oxidative phosphorylation is a process that occurs in the inner mitochondrial membrane where the energy released during the breakdown of nutrients is used to generate adenosine triphosphate (ATP), the primary energy currency of the cell. This process involves a series of protein complexes known as the electron transport chain (ETC).
- Mechanism: During oxidative phosphorylation, electrons are transferred through the ETC, and the energy released is used to pump protons (H⁺ ions) across the inner mitochondrial membrane, creating an electrochemical gradient. This gradient is then used by the enzyme ATP synthase to phosphorylate adenosine diphosphate (ADP) to ATP.
Regulation of Reactive Oxygen Species (ROS):
-
- Generation of ROS: Reactive oxygen species (ROS) are molecules containing oxygen with an unpaired electron, making them highly reactive. While mitochondria are crucial for energy production, a small percentage of electrons in the ETC can leak and react with oxygen, forming ROS.
- Antioxidant Defense: Mitochondria have antioxidant defense mechanisms to regulate ROS levels. Enzymes like superoxide dismutase and catalase help neutralize ROS to prevent cellular damage. The balance between ROS generation and antioxidant defenses is critical for maintaining cellular homeostasis.
Participation in Cellular Signaling Pathways:
-
- Calcium Signaling: Mitochondria play a role in calcium signaling, a critical process for regulating various cellular functions. They sequester and release calcium ions, influencing processes such as cell death (apoptosis) and energy metabolism. The dynamic regulation of calcium within mitochondria contributes to cellular responses to external stimuli.
- Apoptosis: Mitochondria are intimately involved in the regulation of apoptosis, or programmed cell death. Changes in the mitochondrial membrane permeability can lead to the release of pro-apoptotic factors, initiating the apoptotic cascade.
- Cellular Energetics: Mitochondria respond to cellular energy demands by adjusting their function. For instance, in response to increased energy requirements, mitochondria can undergo fission to generate more functional units, and fusion to share content and maintain mitochondrial health.
In summary, mitochondria are multifunctional organelles that not only serve as the powerhouse of the cell through oxidative phosphorylation but also play critical roles in regulating ROS levels and participating in various cellular signaling pathways. These functions are interconnected and contribute to the overall health and functioning of the cell. Dysregulation of mitochondrial processes is implicated in various diseases, underscoring the importance of understanding and maintaining mitochondrial function for cellular well-being.
Why Zone TWO and ZONE FIVE heart rate training?
Zone Two and Zone Five training are terms often associated with specific heart rate zones during exercise, particularly in the context of aerobic or cardiovascular training.
These zones are typically defined based on a percentage of an individual’s maximum heart rate.
While they may not be universally defined, I’ll provide a general overview:
Zone Two Training:
-
- Definition: Zone Two training typically corresponds to a moderate intensity, where your heart rate is around 60-70% of your maximum heart rate.
- Mitochondrial Benefits: Zone Two training is considered an aerobic exercise zone.
- It promotes the development and efficiency of aerobic pathways, which rely heavily on mitochondrial function.
- Regular aerobic exercise stimulates mitochondrial biogenesis—the process of creating new mitochondria.
- This can lead to an increase in both mitochondrial density and function.
Zone Five Training:
-
- Definition: Zone Five training is often associated with high-intensity interval training (HIIT) and corresponds to a very high intensity, with heart rates reaching 90-100% of your maximum heart rate during intervals.
- Mitochondrial Benefits: Zone Five training, particularly in the form of HIIT, can induce stress on the mitochondria.
- This stress triggers adaptive responses, including improvements in mitochondrial function and efficiency.
- The intensity of Zone Five training can lead to increased mitochondrial biogenesis and improvements in the oxidative capacity of muscles.
Both Zone Two and Zone Five training contribute to mitochondrial health, but they do so through different mechanisms:
Zone Two Training:
- Enhances mitochondrial function through steady-state, moderate-intensity exercise, promoting the development of the aerobic energy system.
Zone Five Training (SIT… plus HIIT is Zone 4 if recover to Zone 1):
- Promotes mitochondrial adaptation through the stress-induced by high-intensity intervals.
- This can result in improved mitochondrial function, increased mitochondrial density, and enhanced metabolic flexibility.
It’s worth noting that a well-rounded training program often incorporates a mix of different training zones to target various energy systems and provide a comprehensive approach to fitness. Additionally, individual fitness levels, goals, and health conditions should be considered when determining the appropriate training intensity and duration.