
Role of Insulin and Mitochondria in Metabolic Health
Insulin and mitochondria play interconnected roles in maintaining metabolic health. Insulin regulates nutrient utilization and storage, while mitochondria are the cellular powerhouses responsible for energy production. When these systems are impaired, metabolic dysfunctions such as insulin resistance and mitochondrial dysfunction arise, contributing to chronic conditions like type 2 diabetes and obesity.
1. The Role of Insulin
- Nutrient Uptake and Storage:
- Insulin promotes glucose uptake into cells via GLUT4 transporters, particularly in muscle and adipose tissues.
- Insulin suppresses fat breakdown (lipolysis) and promotes fat synthesis (lipogenesis) to store excess energy.
- Regulator of Energy Balance:
- Insulin ensures a balance between energy supply and demand by directing glucose and fat utilization or storage based on nutrient availability.
2. The Role of Mitochondria
- Energy Production:
- Mitochondria generate ATP via oxidative phosphorylation, using substrates like glucose and fatty acids.
- Metabolic Flexibility:
- Healthy mitochondria adapt to varying energy demands by switching between carbohydrate and fat oxidation.
- Reactive Oxygen Species (ROS):
- While producing energy, mitochondria generate ROS, which, at controlled levels, act as signaling molecules.
3. What Goes Wrong: Insulin Resistance and Mitochondrial Dysfunction
A. Impaired Mitochondrial Oxidative Capacity
 Reduced Energy Production:
Reduced Energy Production:
- Dysfunctional mitochondria have a diminished capacity to oxidize glucose and fatty acids, leading to energy deficits in cells.
- Accumulation of Lipids:
- Reduced fatty acid oxidation leads to intramyocellular lipid accumulation, which contributes to the production of lipotoxic species like ceramide and diacylglycerol.
- Lipotoxicity:
- Ceramide and diacylglycerol interfere with insulin signaling by disrupting the phosphorylation of insulin receptor substrates (IRS), a key step in the insulin signaling pathway.
B. Excess ROS Production
- Oxidative Stress:
- Elevated nutrient supply and impaired mitochondrial function lead to excess ROS, overwhelming the cell’s antioxidant defenses.
- Insulin Resistance:
- ROS directly impair insulin signaling by inducing oxidative damage to proteins involved in the insulin pathway.
- Damage to Mitochondria:
- ROS damage mitochondrial DNA, lipids, and proteins, further impairing mitochondrial function.
C. Mitophagy and Mitochondrial Turnover
- Failure in Mitophagy:
- Damaged mitochondria are removed through mitophagy. However, excessive damage or impaired mitophagy results in the accumulation of dysfunctional mitochondria.
- Vicious Cycle:
- Dysfunctional mitochondria generate more ROS, leading to further insulin resistance and mitochondrial damage.
4. Why This Happens: The Underlying Causes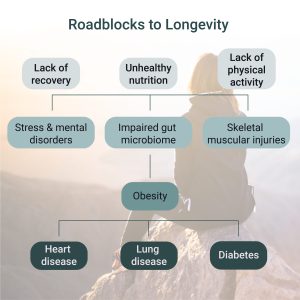
- Overnutrition:
- Chronic overconsumption of calories, especially refined carbohydrates and fats, overwhelms the mitochondria with substrates, increasing ROS production and lipotoxicity.
- Physical Inactivity:
- Reduced physical activity decreases mitochondrial biogenesis and efficiency, impairing the ability to handle metabolic substrates.
- Chronic Inflammation:
- Systemic inflammation, often seen in obesity, damages mitochondria and impairs insulin signaling through pro-inflammatory cytokines.
- Aging:
- Age-related decline in mitochondrial function exacerbates susceptibility to insulin resistance.
- Genetic Predisposition:
- Certain genetic factors may predispose individuals to impaired mitochondrial function or insulin resistance.
5. Strategies to Address Mitochondrial Dysfunction and Insulin Resistance
- Enhancing Mitochondrial Health:
- Exercise: Both aerobic and resistance training improve mitochondrial biogenesis and efficiency.
- Dietary Interventions: Low-carbohydrate, high-fat diets and fasting promote fat oxidation and reduce ROS.
- Supplements: Nutrients like CoQ10, alpha-lipoic acid, and magnesium support mitochondrial function.
- Improving Insulin Sensitivity:
- Weight Loss: Reduces ectopic fat and systemic inflammation.
- Nutrient Timing: Spacing meals and incorporating fasting reduces insulin demand.
- Stress Management: Lowers cortisol, which antagonizes insulin action.
- Addressing ROS and Inflammation:
- Antioxidants: Support the neutralization of excess ROS.
- Anti-inflammatory Foods: Omega-3 fatty acids, turmeric, and polyphenols reduce inflammation.
6. Summary
- Insulin and mitochondria are crucial regulators of metabolic health. Dysfunction in either system creates a cascade of metabolic disturbances.
- Mitochondrial dysfunction reduces the capacity to oxidize fatty acids and glucose, promoting lipotoxicity and insulin resistance.
- Insulin resistance, in turn, exacerbates mitochondrial dysfunction by impairing energy substrate utilization and increasing ROS production.
- Addressing both mitochondrial dysfunction and insulin resistance through lifestyle changes, diet, exercise, and targeted supplementation can help restore metabolic health and prevent chronic diseases.
The Role of Insulin in the Body and Its Connection to Metabolic Health
Insulin is a crucial hormone regulating energy metabolism and blood sugar levels, but modern dietary and lifestyle habits often lead to insulin resistance. Dr. Ben Bikman, in his book Why We Get Sick, explains how insulin resistance lies at the core of many chronic diseases and offers actionable steps to reverse it and enhance long-term health.
Role of Insulin in the Body
- Blood Sugar Regulation: Insulin helps cells absorb glucose from the bloodstream, maintaining stable blood sugar levels.
- Energy Storage: Promotes storage of excess glucose as glycogen in the liver and muscles or as fat in adipose tissue.
- Fat Metabolism: Suppresses fat breakdown (lipolysis) and promotes fat storage (lipogenesis) during high insulin levels.
- Growth and Repair: Supports protein synthesis and cell growth.
Connections to Insulin Resistance and Metabolic Health
- What Goes Wrong in Insulin Resistance:
- Cells stop responding to insulin, leading to elevated blood glucose and insulin levels.
- Over time, this disrupts energy metabolism, promotes inflammation, and contributes to chronic diseases.
- Root Causes of Insulin Resistance:
- Chronic high-carbohydrate and processed food consumption.
- Sedentary lifestyle and lack of muscle activity.
- Chronic stress and poor sleep, elevating cortisol and impairing insulin sensitivity.
- Health Consequences:
- Metabolic Disorders: Type 2 diabetes, obesity, and non-alcoholic fatty liver disease.
- Cardiovascular Diseases: Elevated blood pressure, cholesterol imbalances, and atherosclerosis.
- Chronic Inflammation: A trigger for autoimmune conditions and degenerative diseases.
- Neurodegenerative Diseases: Links to Alzheimer’s disease, now referred to as “Type 3 diabetes.”
How to Improve Insulin Sensitivity
Dr. Bikman emphasizes addressing insulin resistance to prevent and reverse chronic diseases, improve metabolic health, and enhance longevity.
- Adopt a Low-Carb, Healthy Fat Diet:
- Reduce processed carbohydrates and sugars.
- Focus on whole, nutrient-dense foods rich in healthy fats, fiber, and protein.
- Prioritize Resistance Training and Physical Activity:
- Exercise improves glucose uptake by muscles and enhances insulin sensitivity.
- Incorporate Intermittent Fasting:
- Periods of fasting lower insulin levels and improve metabolic flexibility.
- Optimize Sleep and Manage Stress:
- Poor sleep and chronic stress increase cortisol, impairing insulin sensitivity.
- Consider Supplements for Metabolic Support:
- Magnesium, omega-3 fatty acids, and alpha-lipoic acid can enhance insulin function.
- Monitor and Minimize Chronic Inflammation:
- Anti-inflammatory foods like fatty fish, leafy greens, and berries support overall health.
Main Takeaways
- Insulin is essential for energy regulation and metabolic health but becomes detrimental when chronically elevated.
- Insulin resistance is a root cause of many chronic diseases, including diabetes, obesity, and neurodegenerative conditions.
- Simple, sustainable lifestyle changes, as advocated by Dr. Bikman, can reverse insulin resistance, promoting a longer, healthier life.
By understanding insulin’s role and addressing its dysregulation, you can build a foundation for lifelong health and resilience.
Look at the ROOT CAUSE approach instead of a bandaid.
Side Effects of Metformin in Functional Medicine
Metformin is a widely used medication for managing type 2 diabetes. In functional medicine, while its benefits are acknowledged, potential side effects are scrutinized for their systemic impact.
1. Common Side Effects
- Gastrointestinal Distress:
- Nausea, diarrhea, bloating, abdominal pain, and loss of appetite.
- These are often dose-dependent and diminish over time.
- Lactic Acidosis:
- Rare but serious, lactic acidosis occurs due to impaired mitochondrial function and is more common in individuals with kidney or liver dysfunction.
2. Nutritional Implications
- Vitamin B12 Deficiency:
- Long-term use can impair vitamin B12 absorption, potentially leading to deficiency, anemia, and neurological issues.
3. Gut Microbiome Impact
- Altered Microbiota:
- Metformin changes gut microbial composition, which may improve glucose control but also cause GI symptoms or imbalances.
4. Mitochondrial Effects
- Impaired Mitochondrial Function:
- Metformin reduces hepatic gluconeogenesis by inhibiting mitochondrial respiratory chain complex I, which can contribute to reduced energy metabolism in some tissues.
5. Functional Medicine Considerations
- Focus on Root Causes:
- Functional medicine emphasizes improving insulin sensitivity and metabolic health through lifestyle changes, diet, and targeted supplements before or alongside medications.
Side Effects of Statin Drugs in Functional Medicine
Statins are commonly prescribed to lower cholesterol and reduce cardiovascular risk. However, functional medicine practitioners often explore their side effects and long-term systemic impact.
1. Common Side Effects
- Muscle Symptoms:
- Myopathy, muscle pain, weakness, or cramping (statin-associated muscle symptoms or SAMS).
- Rarely, rhabdomyolysis, a severe breakdown of muscle tissue.
- Liver Enzyme Elevation:
- Statins can increase liver enzymes, indicating potential liver stress or damage.
2. Nutritional Implications
- Coenzyme Q10 (CoQ10) Depletion:
- Statins inhibit HMG-CoA reductase, which also reduces CoQ10 production, essential for mitochondrial function and energy production.
- CoQ10 depletion may exacerbate muscle symptoms and fatigue.
- Vitamin D Deficiency:
- Statins may lower vitamin D levels, potentially impacting immune and bone health.
3. Neurological Effects
- Cognitive Impairment:
- Some patients report memory loss or confusion, though evidence is mixed.
4. Metabolic Effects
- Insulin Resistance:
- Statins may increase blood sugar levels, contributing to a higher risk of type 2 diabetes, especially in predisposed individuals.
5. Functional Medicine Considerations
- Addressing Root Causes:
- Focus on dietary changes, inflammation reduction, and stress management to improve lipid profiles naturally.
- Supporting Nutritional Deficiencies:
- CoQ10 supplementation is often recommended to counteract mitochondrial effects.
- Assessing Risk-Benefit:
- Statins are prescribed judiciously, considering individual risk factors and exploring alternatives such as niacin, omega-3 fatty acids, or red yeast rice.
Conclusion
In functional medicine, the side effects of metformin and statins are carefully evaluated. Strategies prioritize addressing root causes of metabolic dysfunction and cardiovascular risk through lifestyle interventions, tailored nutrition, and targeted supplementation to minimize reliance on pharmaceuticals while optimizing long-term health.
The interplay between insulin resistance, mitochondrial dysfunction, and metabolic health in the context of obesity is a central theme in functional medicine. This relationship highlights how disruptions in cellular and molecular mechanisms contribute to chronic metabolic disorders like type 2 diabetes mellitus (T2DM) and other obesity-associated conditions. Below is an explanation of their interconnected roles:
1. Insulin Signaling Pathway and Resistance
Normal Function:
- Insulin is a hormone that regulates glucose uptake in tissues, particularly in skeletal muscle, liver, and adipose tissue.
- Binding of insulin to its receptor triggers a cascade of intracellular signaling through pathways like PI3K/AKT. This enhances glucose transport into cells (via GLUT4) and promotes anabolic processes such as glycogen synthesis.
Insulin Resistance:
- Obesity-induced chronic inflammation (from visceral adiposity) releases pro-inflammatory cytokines (e.g., TNF-α, IL-6), disrupting insulin signaling by increasing serine phosphorylation of insulin receptor substrates (IRS).
- Ectopic fat storage and lipotoxicity in skeletal muscle and liver result in the accumulation of lipid metabolites such as ceramides and diacylglycerols (DAGs). These impair insulin signaling by activating protein kinase C (PKC), which inhibits IRS function.
- Hyperinsulinemia occurs as the pancreas compensates for reduced insulin sensitivity, further exacerbating metabolic dysfunction.
2. Mitochondrial Dysfunction
Role of Mitochondria:
- Mitochondria are critical for energy production via oxidative phosphorylation (OXPHOS) and for the oxidation of fatty acids (β-oxidation).
- Skeletal muscle relies heavily on mitochondria to oxidize fatty acids and glucose, balancing energy production and lipid homeostasis.
Dysfunction in Obesity:
- Obesity and chronic overnutrition overwhelm mitochondrial capacity, leading to reduced fatty acid β-oxidation.
- Incomplete oxidation of fatty acids contributes to the accumulation of lipotoxic intermediates (e.g., ceramides, DAGs).
- Impaired mitochondrial function disrupts ATP production, leading to oxidative stress and excessive reactive oxygen species (ROS) generation. ROS further damages mitochondrial DNA, proteins, and lipids, perpetuating dysfunction.
3. Connection Between Mitochondrial Dysfunction and Insulin Resistance
- Lipotoxicity and ROS: Accumulation of lipid intermediates (ceramides, DAGs) impairs insulin signaling, while ROS exacerbates cellular inflammation.
- Decreased Mitochondrial Biogenesis: Mitochondria fail to adapt to increased energy demands. Reduced levels of PGC-1α, a regulator of mitochondrial biogenesis, are observed in insulin resistance.
- Impaired Fuel Oxidation: An inability to oxidize fatty acids leads to ectopic fat deposition in tissues like liver and muscle, worsening metabolic dysfunction.
4. Role of Dietary and Lifestyle Interventions
Functional medicine emphasizes strategies to address mitochondrial dysfunction and insulin resistance through targeted interventions:
Nutrients and Compounds:
- Omega-3 Fatty Acids: Reduce inflammation and improve mitochondrial function.
- Polyphenols (e.g., resveratrol, quercetin): Enhance mitochondrial biogenesis via activation of PGC-1α and sirtuins.
- Micronutrients (e.g., magnesium, coenzyme Q10): Support OXPHOS and reduce oxidative stress.
Dietary Patterns:
- Low-Glycemic Diets: Prevent hyperglycemia and excessive insulin secretion, reducing metabolic stress on mitochondria.
- Time-Restricted Eating: Improves insulin sensitivity and promotes autophagy, aiding mitochondrial quality control.
Exercise:
- Aerobic Exercise: Enhances mitochondrial biogenesis and fatty acid oxidation, reducing lipotoxicity.
- Resistance Training: Improves insulin sensitivity and muscle glucose uptake.
5. Conclusion
The connection between insulin resistance, mitochondrial dysfunction, and metabolic health illustrates the intricate relationship between cellular energy systems and systemic health. Chronic low-grade inflammation, lipotoxicity, and oxidative stress are central mechanisms in this dysfunction. Functional medicine approaches, which prioritize personalized nutrition, targeted supplementation, and lifestyle modifications, aim to restore mitochondrial function and metabolic flexibility, ultimately addressing the root causes of obesity and its complications.
What does Dr. Ben Bikman think?
Dr. Ben Bikman, a leading researcher and advocate for metabolic health, and his Insulin IQ program provide a clear and science-based explanation of the role of insulin, insulin resistance, and strategies to improve insulin sensitivity. Below is a summary of his perspectives:
1. The Role of Insulin in the Body
Dr. Bikman emphasizes that insulin is a vital hormone for metabolism, with several key functions:
- Regulating Blood Sugar: Insulin facilitates the uptake of glucose from the bloodstream into cells, particularly in muscle and fat tissues.
- Energy Storage: Insulin promotes the storage of excess glucose as glycogen in the liver and muscle or as fat in adipose tissue.
- Inhibiting Lipolysis: Insulin suppresses the breakdown of fat (lipolysis) in adipose tissue, favoring fat storage when insulin levels are high.
- Protein Synthesis: Insulin supports muscle growth and repair by facilitating amino acid uptake into cells.
While insulin is necessary, chronically elevated insulin levels, often due to poor dietary and lifestyle choices, can lead to insulin resistance and metabolic disorders.
2. Insulin Resistance: The Central Issue
Dr. Bikman describes insulin resistance as a state where cells become less responsive to insulin’s effects, leading to metabolic dysfunction. Key points include:
- Causes of Insulin Resistance:
- Chronic High Insulin Levels: Prolonged overproduction of insulin from frequent carbohydrate-heavy meals and snacking.
- Ectopic Fat Storage: Accumulation of fat in liver and muscle tissues impairs insulin signaling.
- Chronic Inflammation: Inflammatory markers interfere with insulin receptor function.
- Mitochondrial Dysfunction: Impaired energy production contributes to the buildup of lipid intermediates that disrupt insulin signaling.
- Effects of Insulin Resistance:
- Elevated Blood Glucose Levels: As cells resist insulin, glucose remains in the bloodstream.
- Hyperinsulinemia: The pancreas compensates by producing more insulin, exacerbating the issue.
- Metabolic Disorders: Insulin resistance is a precursor to type 2 diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), and cardiovascular disease.
3. Improving Insulin Sensitivity
Dr. Bikman’s Insulin IQ program provides practical strategies to enhance insulin sensitivity by targeting root causes. These include:
Dietary Recommendations
- Low-Carbohydrate, High-Fat (LCHF) Diet:
- Reduces glucose and insulin spikes.
- Encourages the body to rely on fat (ketones) for energy.
- Prioritize Protein:
- Adequate protein supports muscle repair and satiety without excessively stimulating insulin.
- Avoid Processed Carbs and Sugars:
- Refined carbohydrates rapidly raise blood sugar and insulin, promoting insulin resistance.
Fasting and Meal Timing
- Intermittent Fasting: Periods of fasting lower insulin levels and improve insulin sensitivity.
- Time-Restricted Eating: Aligning meals with circadian rhythms enhances metabolic health.
Exercise
- Resistance Training:
- Increases muscle mass, which acts as a glucose sink and improves insulin sensitivity.
- Aerobic Exercise:
- Enhances mitochondrial function and fatty acid oxidation.
Lifestyle Modifications
- Sleep Optimization:
- Poor sleep is linked to increased insulin resistance and impaired glucose regulation.
- Stress Management:
- Chronic stress elevates cortisol, which antagonizes insulin action.
Supplementation
Dr. Bikman supports evidence-based supplements to improve insulin sensitivity, such as:
- Magnesium: Supports insulin receptor function.
- Berberine: Mimics insulin and reduces blood sugar levels.
- Alpha-Lipoic Acid: Enhances glucose metabolism and reduces oxidative stress.

4. The Key Philosophy of Insulin IQ
Dr. Bikman emphasizes that metabolic health is the foundation of overall health, and insulin plays a central role. By focusing on strategies to keep insulin levels balanced and improving the body’s sensitivity to insulin, individuals can:
- Reverse metabolic disorders.
- Enhance energy levels and cognitive function.
- Reduce the risk of chronic diseases.
- Achieve sustainable weight management.
The Insulin IQ program integrates scientific principles with practical advice to empower individuals to take control of their metabolic health. Dr. Bikman’s message is that understanding and optimizing insulin function is one of the most impactful steps to achieving long-term health.
https://www.insuliniq.com/eat-insulin-smart0
The Role of NAD in Mitochondrial Health, Insulin Sensitivity, Hormones, Metabolic Health, and Aging
Nicotinamide adenine dinucleotide (NAD⁺) is a critical coenzyme in cellular energy production and metabolic processes. It plays a pivotal role in mitochondrial function, insulin sensitivity, hormone regulation, metabolic health, and aging. Declining NAD⁺ levels, often associated with aging and chronic metabolic dysfunction, disrupt these interconnected systems, contributing to many chronic diseases.
How NAD⁺ Impacts Metabolic and Hormonal Health
1. Mitochondrial Health
- Energy Production: NAD⁺ is essential for oxidative phosphorylation, where mitochondria generate ATP, the cell’s energy currency.
- Mitochondrial Maintenance: Supports mitochondrial biogenesis and turnover (via mitophagy), ensuring efficient energy metabolism.
- Antioxidant Defense: Reduces mitochondrial reactive oxygen species (ROS) production and oxidative damage, preserving mitochondrial DNA and proteins.
2. Insulin Sensitivity
- Glucose Uptake: NAD⁺ is required for enzymes involved in glucose metabolism, improving cellular glucose uptake and reducing insulin resistance.
- Lipid Metabolism: Enhances fatty acid oxidation, preventing the accumulation of lipotoxic intermediates like ceramides and diacylglycerol, which impair insulin signaling.
3. Hormone Regulation
- Adrenal Function: Influences the production of cortisol and other stress-related hormones.
- Sex Hormones: Impacts testosterone and estrogen production through its role in cellular repair and metabolic regulation.
- Thyroid Hormones: Optimizes mitochondrial function necessary for thyroid hormone activation and energy balance.
4. Metabolic Health
- Inflammation Control: Activates sirtuins (SIRT1–7), a family of proteins that regulate inflammation and metabolic processes.
- Fat Storage and Mobilization: Enhances fat breakdown (lipolysis) and prevents ectopic fat deposition in the liver and muscle.
- Caloric Restriction Mimicry: NAD⁺ levels increase during fasting or caloric restriction, enhancing metabolic flexibility and reducing metabolic stress.
5. Aging and Longevity
- DNA Repair: NAD⁺ is required for poly(ADP-ribose) polymerases (PARPs), which repair DNA damage, a key factor in cellular aging.
- Epigenetic Regulation: Sirtuins, activated by NAD⁺, regulate genes involved in aging and stress resistance.
- Cellular Senescence: Maintains the health and function of aging cells by improving mitochondrial function and reducing oxidative stress.
What Goes Wrong: NAD⁺ Decline and Its Consequences
- Aging: NAD⁺ levels decline naturally with age, impairing mitochondrial function and increasing oxidative stress.
- Chronic Metabolic Stress: Insulin resistance, obesity, and inflammation deplete NAD⁺, worsening mitochondrial dysfunction and metabolic inflexibility.
- Hormonal Imbalance: Low NAD⁺ impairs the synthesis and regulation of critical hormones, including cortisol, thyroid hormones, and sex hormones.
- Mitochondrial Dysfunction: Reduced NAD⁺ limits energy production, exacerbating fatigue, metabolic disorders, and aging processes.
How to Boost NAD⁺ Levels
 Nutritional Support:
Nutritional Support:
- NAD⁺ Precursors: Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) supplements can directly increase NAD⁺ levels.
- Polyphenols: Found in foods like berries, green tea, and dark chocolate, polyphenols enhance NAD⁺ synthesis and sirtuin activation.
- Fasting and Caloric Restriction:
- Increases NAD⁺ levels by reducing its consumption in energy-intensive processes.
- Exercise:
- Boosts NAD⁺ through enhanced mitochondrial biogenesis and turnover.
- Optimizing Sleep and Stress:
- Reduces the NAD⁺ drain caused by chronic stress and poor recovery.
- Supplemental Support:
- CoQ10 and Resveratrol: Support mitochondrial health and complement NAD⁺-boosting strategies.
Key Takeaways
- NAD⁺ is essential for mitochondrial function, insulin sensitivity, hormone regulation, metabolic health, and slowing the aging process.
- A decline in NAD⁺ disrupts these systems, leading to metabolic dysfunction, hormonal imbalances, and accelerated aging.
- Lifestyle interventions, dietary strategies, and targeted supplementation can restore NAD⁺ levels, promoting metabolic resilience, hormonal balance, and longevity.
By integrating NAD⁺ optimization into functional medicine strategies, we can address critical missing pieces of the puzzle in metabolic health, aging, and chronic disease prevention.
The Role of Insulin in the Body and Its Connection to Metabolic Health
Insulin is a crucial hormone regulating energy metabolism and blood sugar levels, but modern dietary and lifestyle habits often lead to insulin resistance. Dr. Ben Bikman, in his book Why We Get Sick, explains how insulin resistance lies at the core of many chronic diseases and offers actionable steps to reverse it and enhance long-term health.
Role of Insulin in the Body
- Blood Sugar Regulation: Insulin helps cells absorb glucose from the bloodstream, maintaining stable blood sugar levels.
- Energy Storage: Promotes storage of excess glucose as glycogen in the liver and muscles or as fat in adipose tissue.
- Fat Metabolism: Suppresses fat breakdown (lipolysis) and promotes fat storage (lipogenesis) during high insulin levels.
- Growth and Repair: Supports protein synthesis and cell growth.
Connections to Insulin Resistance and Metabolic Health
- What Goes Wrong in Insulin Resistance:
- Cells stop responding to insulin, leading to elevated blood glucose and insulin levels.
- Over time, this disrupts energy metabolism, promotes inflammation, and contributes to chronic diseases.
- Root Causes of Insulin Resistance:
- Chronic high-carbohydrate and processed food consumption.
- Sedentary lifestyle and lack of muscle activity.
- Chronic stress and poor sleep, elevating cortisol and impairing insulin sensitivity.
- Health Consequences:
- Metabolic Disorders: Type 2 diabetes, obesity, and non-alcoholic fatty liver disease.
- Cardiovascular Diseases: Elevated blood pressure, cholesterol imbalances, and atherosclerosis.
- Chronic Inflammation: A trigger for autoimmune conditions and degenerative diseases.
- Neurodegenerative Diseases: Links to Alzheimer’s disease, now referred to as “Type 3 diabetes.”
How to Improve Insulin Sensitivity
Dr. Bikman emphasizes addressing insulin resistance to prevent and reverse chronic diseases, improve metabolic health, and enhance longevity.
- Adopt a Low-Carb, Healthy Fat Diet:
- Reduce processed carbohydrates and sugars.
- Focus on whole, nutrient-dense foods rich in healthy fats, fiber, and protein.
- Prioritize Resistance Training and Physical Activity:
- Exercise improves glucose uptake by muscles and enhances insulin sensitivity.
- Incorporate Intermittent Fasting:
- Periods of fasting lower insulin levels and improve metabolic flexibility.
- Optimize Sleep and Manage Stress:
- Poor sleep and chronic stress increase cortisol, impairing insulin sensitivity.
- Consider Supplements for Metabolic Support:
- Magnesium, omega-3 fatty acids, and alpha-lipoic acid can enhance insulin function.
- Monitor and Minimize Chronic Inflammation:
- Anti-inflammatory foods like fatty fish, leafy greens, and berries support overall health.
Main Takeaways
- Insulin is essential for energy regulation and metabolic health but becomes detrimental when chronically elevated.
- Insulin resistance is a root cause of many chronic diseases, including diabetes, obesity, and neurodegenerative conditions.
- Simple, sustainable lifestyle changes, as advocated by Dr. Bikman, can reverse insulin resistance, promoting a longer, healthier life.
By understanding insulin’s role and addressing its dysregulation, you can build a foundation for lifelong health and resilience.
