What is Oxidative Stress?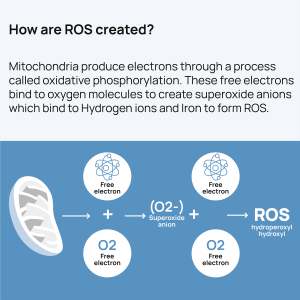
Oxidative stress refers to an imbalance between the production of free radicals (reactive oxygen species, ROS) and the body’s ability to counteract or detoxify their harmful effects through antioxidants. Free radicals are highly reactive molecules with unpaired electrons that can damage cells, proteins, and DNA by oxidation. Antioxidants are substances that can donate an electron to neutralize free radicals without becoming destabilized themselves.
Oxidative Stress in Endurance Athletes and Aging Individuals
Endurance Athletes:
Endurance exercise increases oxygen consumption, which can elevate the production of ROS. While moderate levels of ROS are beneficial for physiological adaptations such as improved muscle function and enhanced endurance capacity, excessive ROS can lead to oxidative damage if the antioxidant defense system is overwhelmed. This oxidative stress can affect liver function by increasing the load on the liver’s detoxification processes, potentially leading to liver congestion. However, well-trained athletes often have adaptive mechanisms that enhance their antioxidant defenses.
Aging Individuals:
Aging is associated with a natural decline in the efficiency of the body’s antioxidant defense mechanisms and an increase in ROS production. This results in a higher baseline level of oxidative stress.
The liver, being a primary organ for detoxification, can become overwhelmed as its capacity to process and eliminate toxins diminishes with age. This can contribute to liver congestion and decreased detoxification efficiency.
Role of Oxidative Stress and Glutathione
Oxidative Stress:
- Cell Damage: Oxidative stress can cause lipid peroxidation (damage to cell membranes), protein oxidation (affecting enzyme function), and DNA damage (increasing mutation rates and cancer risk).
- Inflammation: It can activate inflammatory pathways, leading to chronic inflammation and associated diseases such as cardiovascular disease, diabetes, and neurodegenerative disorders.
Glutathione:
Glutathione is a tripeptide composed of glutamine, cysteine, and glycine. It is one of the most important intracellular antioxidants and plays a critical role in reducing oxidative stress by:
- Direct Neutralization of Free Radicals: Glutathione can directly neutralize free radicals and reactive oxygen species.
- Regeneration of Other Antioxidants: It helps regenerate other antioxidants like vitamins C and E back to their active forms.
- Detoxification: Glutathione conjugates with toxins to make them more water-soluble, facilitating their excretion via the liver. This process is crucial for detoxifying harmful substances and preventing liver congestion.
- Immune Function: It supports the immune system by maintaining the function of lymphocytes and other immune cells.
Impact on Liver Detox Congestion
Both endurance athletes and aging individuals can experience increased oxidative stress, which places additional demands on the liver’s detoxification system. The liver requires adequate levels of glutathione to efficiently process and eliminate toxins.
When oxidative stress is high:
-
Glutathione Depletion: Excessive ROS can deplete glutathione levels, impairing the liver’s ability to detoxify effectively.
-
Increased Detoxification Demand: The liver has to work harder to neutralize and eliminate the increased load of oxidative byproducts and toxins, potentially leading to congestion and reduced detoxification capacity.
-
Inflammation and Damage: Chronic oxidative stress can cause liver cell damage and inflammation, further impairing liver function and contributing to a cycle of increasing oxidative stress and liver congestion.
In summary, managing oxidative stress through adequate nutrition, antioxidant intake, and lifestyle modifications is crucial for both endurance athletes and aging individuals to maintain liver health and overall well-being.
Do you know how much oxidative stress we create when we exercise?
My recent discovery from learning from the recent HACK YOUR HEALTH conference.
Experience the Power of Topical Glutathione
A CELLULAR BREAKTHROUGH TO MANAGE STRESS AND TOXINS
What is our Master Antioxidant?
Glutathione (GSH) is a naturally occurring “Master Antioxidant” vital for overall health. It is the body’s first line of defense against free radical stressors our bodies encounter daily, like pollution, UV rays, chemicals, and more.
However, aging and oxidative stress lessen our Glutathione levels.
Glutaryl+, the patented antioxidant spray, delivers a highly absorbable form of reduced L-glutathione utilizing groundbreaking sub-nanotechnology that allows the reduced glutathione molecule to absorb into your skin.
Auro Wellness products enhance health and vitality to promote proper energy levels and healthy aging. The patented antioxidant sprays Glutaryl and Glutaryl+ harness the power of the naturally occurring Master Antioxidant Glutathione so the skin can absorb it more potently than ever before with the breakthrough Auro GSH™ Antioxidant Delivery System. It delivers antioxidants like Glutathione to the skin with sub-nano technology stabilizing this powerful ingredient to dramatically increase effectiveness. Auro Wellness nutritional supplements support the vitality of the body’s many functions, including digestive, adrenal, and neurological functions and healthy stress response and energy levels.
This formula contains the highest levels of Glutathione to jumpstart your body’s Glutathione levels.
This dosage is recommended to help athletes or any individual under high oxidative stress to recover.
These dosages can also be used to kickstart your journey and then go down.
Our Wellness products have been third-party lab tested, and are Non-Comedogenic and Hypoallergenic.
They are also free of parabens, sulfates, sulfates, perfumes, mineral oil, artificial preservatives, artificial colors, gluten, and dairy. They are cruelty-free. The products are only made with the cleanest ingredients including the most purified water.
I started using Auro Wellness Gluathione spray last month morning and evening.
Learn more on an upcoming podcast plus research on their website but save with our discount code COACHDEBBIEPOTTS if you make a purchase!
How much oxidative stress is created in exercise during various intensities?


